High frequency electrical stimulation induces a long-lasting enhancement of event-related potentials but does not change the perception elicited by intra-epidermal electrical stimuli delivered to the area of increased mechanical pinprick sensitivity
- PMID: 30188910
- PMCID: PMC6126845
- DOI: 10.1371/journal.pone.0203365
High frequency electrical stimulation induces a long-lasting enhancement of event-related potentials but does not change the perception elicited by intra-epidermal electrical stimuli delivered to the area of increased mechanical pinprick sensitivity
Abstract
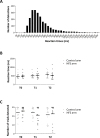
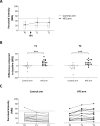
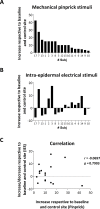
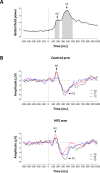
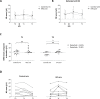
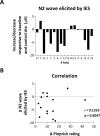
High frequency electrical stimulation (HFS) of the skin induces increased pinprick sensitivity in the surrounding unconditioned skin. The aim of the present study was to investigate the contribution of A-fiber nociceptors to this increased pinprick sensitivity. For this we assessed if the perception and brain responses elicited by low-intensity intra-epidermal electrical stimulation (IES), a method preferentially activating Aδ-fiber nociceptors, are increased in the area of HFS-induced increased pinprick sensitivity. HFS was delivered to one of the two forearms of seventeen healthy volunteers. Mechanical pinprick stimulation and IES were delivered at both arms before HFS (T0), 20 minutes after HFS (T1) and 45 minutes after HFS (T2). In all participants, HFS induced an increase in pinprick perception at the HFS-treated arm, adjacent to the site of HFS. This increase was significant at both T1 and T2. HFS did not affect the percept elicited by IES, but did enhance the magnitude of the N2 wave of IES-evoked brain potentials, both at T1 and at T2. Our results show that HFS induces a long-lasting enhancement of the N2 wave elicited by IES in the area of secondary hyperalgesia, indicating that HFS enhances the responsiveness of the central nervous system to nociceptive A-fiber input. However, we found no evidence that HFS affects the perception elicited by IES, which may suggest that the population of nociceptors that mediate the perception elicited by IES do not contribute to HFS-induced increased pinprick sensitivity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Enhanced brain responses to C-fiber input in the area of secondary hyperalgesia induced by high-frequency electrical stimulation of the skin.J Neurophysiol. 2014 Nov 1;112(9):2059-66. doi: 10.1152/jn.00342.2014. Epub 2014 Aug 6. J Neurophysiol. 2014. PMID: 25098966 Free PMC article.
-
High-frequency electrical stimulation of the human skin induces heterotopical mechanical hyperalgesia, heat hyperalgesia, and enhanced responses to nonnociceptive vibrotactile input.J Neurophysiol. 2014 Apr;111(8):1564-73. doi: 10.1152/jn.00651.2013. Epub 2014 Jan 22. J Neurophysiol. 2014. PMID: 24453277
-
Secondary hyperalgesia is mediated by heat-insensitive A-fibre nociceptors.J Physiol. 2016 Nov 15;594(22):6767-6776. doi: 10.1113/JP272599. Epub 2016 Aug 2. J Physiol. 2016. PMID: 27377467 Free PMC article.
-
Brain potentials evoked by intraepidermal electrical stimuli reflect the central sensitization of nociceptive pathways.J Neurophysiol. 2016 Aug 1;116(2):286-95. doi: 10.1152/jn.00013.2016. Epub 2016 Apr 20. J Neurophysiol. 2016. PMID: 27098022 Free PMC article.
-
[Clinical application of pain-related evoked potentials].Schmerz. 2012 Feb;26(1):8-15. doi: 10.1007/s00482-011-1117-1. Schmerz. 2012. PMID: 22134376 Review. German.
Cited by
-
Assessing signs of central sensitization: A critical review of physiological measures in experimentally induced secondary hyperalgesia.Eur J Pain. 2025 Mar;29(3):e4733. doi: 10.1002/ejp.4733. Epub 2024 Sep 24. Eur J Pain. 2025. PMID: 39315535 Free PMC article. Review.
-
Human surrogate models of central sensitization: A critical review and practical guide.Eur J Pain. 2021 Aug;25(7):1389-1428. doi: 10.1002/ejp.1768. Epub 2021 May 8. Eur J Pain. 2021. PMID: 33759294 Free PMC article.
-
Extensive sensorimotor training enhances nociceptive cortical responses in healthy individuals.Eur J Pain. 2023 Feb;27(2):257-277. doi: 10.1002/ejp.2057. Epub 2022 Dec 1. Eur J Pain. 2023. PMID: 36394423 Free PMC article.
-
A back-translational study of descending interactions with the induction of hyperalgesia by high-frequency electrical stimulation in rats and humans.Pain. 2024 Sep 1;165(9):1978-1989. doi: 10.1097/j.pain.0000000000003166. Epub 2024 Jan 9. Pain. 2024. PMID: 38198231 Free PMC article.
-
Distress is positively associated with induced secondary hyperalgesia in people with suppressed HIV.medRxiv [Preprint]. 2025 Apr 30:2025.01.27.25321015. doi: 10.1101/2025.01.27.25321015. medRxiv. 2025. Update in: Pain. 2025 Aug 7. doi: 10.1097/j.pain.0000000000003748. PMID: 39974111 Free PMC article. Updated. Preprint.
References
-
- Pfau DB, Klein T, Putzer D, Pogatzki-Zahn EM, Treede R-D, Magerl W. Analysis of hyperalgesia time courses in humans after painful electrical high-frequency stimulation identifies a possible transition from early to late LTP-like pain plasticity. Pain 2011; 152 (7): 1532–1539. 10.1016/j.pain.2011.02.037 - DOI - PubMed
-
- Henrich F, Magerl W, Klein T, Greffrath W, Treede R-D. Capsaicin-sensitive C- and A-fibre nociceptors control long-term potentiation-like pain amplification in humans. Brain 2015; 138 (9): 2505–2520. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

