SMAD1/5 signaling in osteoclasts regulates bone formation via coupling factors
- PMID: 30188920
- PMCID: PMC6126839
- DOI: 10.1371/journal.pone.0203404
SMAD1/5 signaling in osteoclasts regulates bone formation via coupling factors
Abstract
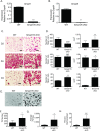
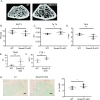
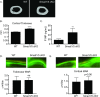
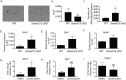
Bone remodeling occurs via coupling between bone resorption by osteoclasts and bone formation by osteoblasts. The mechanisms that regulate osteoclast signals to osteoblasts are not well understood. Published studies have reported that BMP signaling in osteoclasts regulate osteoclast coupling targets. To investigate the necessity of canonical BMP signaling on osteoclast differentiation and coupling, we mated Smad1fl/fl; Smad5fl/fl mice to c-Fms-Cre mice. We analyzed male mice at 3 months of age to determine the skeletal phenotype of the Smad1fl/fl; Smad5fl/fl;c-Fms-Cre (SMAD1/5 cKO) mice. There was a 1.2-fold decrease in trabecular BV/TV in SMAD1/5 cKO. Analyses of osteoclast serum markers in SMAD1/5 cKO mice, showed a significant increase in CTX-1 (1.5 fold) and TRAP ELISA (3 fold) compared to control mice. In these same mice, there was a 1.3-fold increase in cortical thickness. Consistent with the increase in cortical thickness, we found a 3-fold increase in osteoblast activity as measured by P1NIP ELISA assay from SMAD1/5 cKO mice. To explain the changes in cortical thickness and P1NP activity, we determined conditioned media from SMAD1/5 cKO osteoclast cultures enhanced mineralization of an osteoblast cell line and coupling factors expressed by osteoclasts that regulate osteoblast activity Wnt1 (4.5-fold increase), Gja1 (3-fold increase) and Sphk1 (1.5-fold increase) were all upregulated in osteoclasts from SMAD1/5 cKO compared to control osteoclasts. Lastly osteoclasts treated with dorsomorphin, a chemical inhibitor of SMAD1/5 signaling, demonstrates an increase in Wnt1 and Gja1 expression similar to the SMAD1/5 cKO mice. Previous studies demonstrated that TGF-β signaling in osteoclasts leads to increases in WNT1 expression by osteoclasts. Therefore, our data suggest that TGF-β and BMP signaling pathways in osteoclasts could act in an antagonistic fashion to regulate osteoblast activity through WNT1 and other coupling factors.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Broege A, Pham L, Jensen ED, Emery A, Huang TH, Stemig M, et al. Bone morphogenetic proteins signal via SMAD and mitogen-activated protein (MAP) kinase pathways at distinct times during osteoclastogenesis. The Journal of biological chemistry. 2013;288(52):37230–40. 10.1074/jbc.M113.496950 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

