Pharmacodynamic Analysis of Daptomycin-treated Enterococcal Bacteremia: It Is Time to Change the Breakpoint
- PMID: 30188976
- PMCID: PMC6938208
- DOI: 10.1093/cid/ciy749
Pharmacodynamic Analysis of Daptomycin-treated Enterococcal Bacteremia: It Is Time to Change the Breakpoint
Abstract
Background: Currently, there is debate over whether the daptomycin susceptibility breakpoint for enterococci (ie, minimum inhibitory concentration [MIC] ≤4 mg/L) is appropriate. In bacteremia, observational data support prescription of high doses (>8 mg/kg). However, pharmacodynamic targets associated with positive patient outcomes are undefined.
Methods: Data were pooled from observational studies that assessed outcomes in daptomycin-treated enterococcal bacteremia. Patients who received an additional antienterococcal antibiotic and/or a β-lactam antibiotic at any time during treatment were excluded. Daptomycin exposures were calculated using a published population pharmacokinetic model. The free drug area under the concentration-time curve to MIC ratio (fAUC/MIC) threshold predictive of survival at 30 days was identified by classification and regression tree analysis and confirmed with multivariable logistic regression. Monte Carlo simulations determined the probability of target attainment (PTA) at clinically relevant MICs.
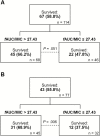
Results: Of 114 patients who received daptomycin monotherapy, 67 (58.8%) were alive at 30 days. A fAUC/MIC >27.43 was associated with survival in low-acuity (n = 77) patients (68.9 vs 37.5%, P = .006), which remained significant after adjusting for infection source and immunosuppression (P = .026). The PTA for a 6-mg/kg/day (every 24 hours) dose was 1.5%-5.5% when the MIC was 4 mg/L (ie, daptomycin-susceptible) and 91.0%-97.9% when the MIC was 1 mg/L.
Conclusions: For enterococcal bacteremia, a daptomycin fAUC/MIC >27.43 was associated with 30-day survival among low-acuity patients. As pharmacodynamics for the approved dose are optimized only when MIC ≤1 mg/L, these data continue to stress the importance of reevaluation of the susceptibility breakpoint.
Keywords: Enterococcus; bacteremia; daptomycin; pharmacodynamics.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


Comment in
-
Concerns About the Association Between Poor Clinical Outcomes and the Minimum Inhibitory Concentrations Determined by Etest.Clin Infect Dis. 2019 Aug 16;69(5):902-903. doi: 10.1093/cid/ciz079. Clin Infect Dis. 2019. PMID: 30715196 No abstract available.
-
Reply to Cheng and Chuang.Clin Infect Dis. 2019 Aug 16;69(5):903-904. doi: 10.1093/cid/ciz081. Clin Infect Dis. 2019. PMID: 30715199 No abstract available.
References
-
- Merck & Co. Inc. Cubicin package insert-US. Kenilworth, New Jersey: Merck & Co. Inc, 2017. Available at: https://www.merck.com/product/usa/pi_circulars/c/cubicin/cubicin_pi.pdf. Accessed 1 May 2018.
-
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. 28th ed. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute, 2018.
-
- Moise PA, Sakoulas G, McKinnell JA, et al. Clinical outcomes of daptomycin for vancomycin-resistant enterococcus bacteremia. Clin Ther 2015; 37:1443–53.e2. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

