Pharmacokinetics, Safety, and Efficacy of an Allometric Miltefosine Regimen for the Treatment of Visceral Leishmaniasis in Eastern African Children: An Open-label, Phase II Clinical Trial
- PMID: 30188978
- PMCID: PMC6481997
- DOI: 10.1093/cid/ciy747
Pharmacokinetics, Safety, and Efficacy of an Allometric Miltefosine Regimen for the Treatment of Visceral Leishmaniasis in Eastern African Children: An Open-label, Phase II Clinical Trial
Abstract
Background: Convenient, safe, and effective treatments for visceral leishmaniasis in Eastern African children are lacking. Miltefosine, the only oral treatment, failed to achieve adequate efficacy, particularly in children, in whom linear dosing (2.5 mg/kg/day for 28 days) resulted in a 59% cure rate, with lower systemic exposure than in adults.
Methods: We conducted a Phase II trial in 30 children with visceral leishmaniasis, aged 4-12 years, to test whether 28 days of allometric miltefosine dosing safely achieves a higher systemic exposure than linear dosing.
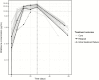
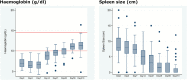
Results: Miltefosine accumulated during treatment. Median areas under the concentration time curve from days 0-210 and plasma maximum concentration values were slightly higher than those reported previously for children on linear dosing, but not dose-proportionally. Miltefosine exposure at the start of treatment was increased, with higher median plasma concentrations on day 7 (5.88 versus 2.67 μg/mL). Concentration-time curves were less variable, avoiding the low levels of exposure observed with linear dosing. The 210-day cure rate was 90% (95% confidence interval, 73-98%), similar to that previously described in adults. There were 19 treatment-related adverse events (AEs), but none caused treatment discontinuation. There were 2 serious AEs: both were unrelated to treatment and both patients were fully recovered.
Conclusions: Allometric miltefosine dosing achieved increased and less-variable exposure than linear dosing, though not reaching the expected exposure levels. The new dosing regimen safely increased the efficacy of miltefosine for Eastern African children with visceral leishmaniasis. Further development of miltefosine should adopt allometric dosing in pediatric patients.
Clinical trials registration: NCT02431143.
Keywords: Eastern African children; allometric regimen; drug pharmacokinetics; miltefosine; visceral leishmaniasis.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Fact sheet–Leishmaniasis. Available at: http://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Accessed 17 April 2017.
-
- World Health Organization. Global leishmaniasis update, 2006–2015: a turning point in leishmaniasis surveillance. WHO Weekly Epidemiological Record 2017; No. 38, 92:557–72. Available at: http://www.who.int/wer.
-
- World Health Organization. Report 949: Control of the leishmaniases. Geneva, Switzerland: World Health Organization, 2010.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

