Harnessing the Noncovalent Interactions of DNA Backbone with 2D Silicate Nanodisks To Fabricate Injectable Therapeutic Hydrogels
- PMID: 30189128
- PMCID: PMC6563937
- DOI: 10.1021/acsnano.8b02434
Harnessing the Noncovalent Interactions of DNA Backbone with 2D Silicate Nanodisks To Fabricate Injectable Therapeutic Hydrogels
Abstract
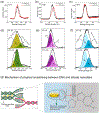
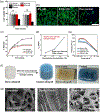
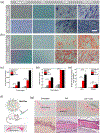
Injectable hydrogels present several advantages over prefabricated scaffolds including ease of delivery, shear-thinning property, and broad applicability in the fields of drug delivery and tissue engineering. Here, we report an approach to develop injectable hydrogels with sustained drug release properties, exploiting the chemical nature of the DNA backbone and silicate nanodisks. A two-step gelation method is implemented for generating a combination of noncovalent network points, leading to a physically cross-linked hydrogel. The first step initiates the development of an interconnected structure by utilizing DNA denaturation and rehybridization mechanism to form hydrogen bonds between complementary base pairs of neighboring DNA strands. The anisotropic charge distribution of two-dimensional silicate nanodisks (nSi) makes them an active center in the second step of the gelation process. Silicate nanodisks create additional network points via attractive electrostatic interactions with the DNA backbone, thereby enhancing the mechanical resilience of the formulated hydrogel. The thermally stable hydrogels displayed an increase in elasticity and yield stress as a function of nSi concentration. They were able to form self-supporting structures post injection due to their rapid recovery after removal of cyclic stress. Moreover, the presence of nanosilicate was shown to modulate the release of a model osteogenic drug dexamethasone (Dex). The bioactivity of released Dex was confirmed from in vitro osteogenic differentiation of human adipose stem cells and in vivo bone formation in a rat cranial bone defect model. Overall, our DNA-based nanocomposite hydrogel obtained from a combination of noncovalent network points can serve as an injectable material for bone regeneration and carrier for sustained release of therapeutics.
Keywords: DNA; controlled release; injectable hydrogels; nanocomposites; physical cross-linking; two-dimensional nanosilicates.
Figures



Similar articles
-
Self-healing DNA-based injectable hydrogels with reversible covalent linkages for controlled drug delivery.Acta Biomater. 2020 Mar 15;105:159-169. doi: 10.1016/j.actbio.2020.01.021. Epub 2020 Jan 20. Acta Biomater. 2020. PMID: 31972367
-
Nucleic Acid-Based Dual Cross-Linked Hydrogels for in Situ Tissue Repair via Directional Stem Cell Migration.ACS Appl Mater Interfaces. 2019 Sep 25;11(38):34621-34633. doi: 10.1021/acsami.9b10074. Epub 2019 Sep 17. ACS Appl Mater Interfaces. 2019. PMID: 31483598 Free PMC article.
-
Engineering natural DNA matrices with halloysite nanotubes to fabricate injectable therapeutic hydrogels for bone regeneration.J Orthop Translat. 2024 Oct 22;49:218-229. doi: 10.1016/j.jot.2024.09.010. eCollection 2024 Nov. J Orthop Translat. 2024. PMID: 39507323 Free PMC article.
-
Spatiotemporal controlled released hydrogels for multi-system regulated bone regeneration.J Control Release. 2024 Aug;372:846-861. doi: 10.1016/j.jconrel.2024.06.065. Epub 2024 Jul 6. J Control Release. 2024. PMID: 38955252 Review.
-
A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration.Int J Biol Macromol. 2019 Jan;121:38-54. doi: 10.1016/j.ijbiomac.2018.10.014. Epub 2018 Oct 4. Int J Biol Macromol. 2019. PMID: 30291931 Review.
Cited by
-
2D Nanoclay for Biomedical Applications: Regenerative Medicine, Therapeutic Delivery, and Additive Manufacturing.Adv Mater. 2019 Jun;31(23):e1900332. doi: 10.1002/adma.201900332. Epub 2019 Apr 3. Adv Mater. 2019. PMID: 30941811 Free PMC article. Review.
-
Recent advance in bioactive hydrogels for repairing spinal cord injury: material design, biofunctional regulation, and applications.J Nanobiotechnology. 2023 Jul 24;21(1):238. doi: 10.1186/s12951-023-01996-y. J Nanobiotechnology. 2023. PMID: 37488557 Free PMC article. Review.
-
Application of Inorganic Nanocomposite Hydrogels in Bone Tissue Engineering.iScience. 2020 Nov 23;23(12):101845. doi: 10.1016/j.isci.2020.101845. eCollection 2020 Dec 18. iScience. 2020. Retraction in: iScience. 2021 Jun 05;24(6):102668. doi: 10.1016/j.isci.2021.102668. PMID: 33305193 Free PMC article. Retracted. Review.
-
Clay-Based Nanocomposite Hydrogels for Biomedical Applications: A Review.Nanomaterials (Basel). 2022 Sep 23;12(19):3308. doi: 10.3390/nano12193308. Nanomaterials (Basel). 2022. PMID: 36234440 Free PMC article. Review.
-
Design and application of stimuli-responsive DNA hydrogels: A review.Mater Today Bio. 2022 Sep 14;16:100430. doi: 10.1016/j.mtbio.2022.100430. eCollection 2022 Dec. Mater Today Bio. 2022. PMID: 36157049 Free PMC article. Review.
References
-
- Park H; Woo EK; Lee KY Ionically Cross-linkable Hyaluronate-based Hydrogels for Injectable Cell Delivery. J. Controlled Release 2014, 196, 146–153. - PubMed
-
- Tan H; Xiao C; Sun J; Xiong D; Hu X Biological Self-assembly of Injectable Hydrogel as Cell Scaffold via Specific Nucleobase Pairing. Chem. Commun. 2012, 48, 10289–10291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

