A Caenorhabditis elegans assay of seizure-like activity optimised for identifying antiepileptic drugs and their mechanisms of action
- PMID: 30189284
- PMCID: PMC6200019
- DOI: 10.1016/j.jneumeth.2018.09.004
A Caenorhabditis elegans assay of seizure-like activity optimised for identifying antiepileptic drugs and their mechanisms of action
Abstract
Background: Epilepsy affects around 1% of people, but existing antiepileptic drugs (AEDs) only offer symptomatic relief and are ineffective in approximately 30% of patients. Hence, new AEDs are sorely needed. However, a major bottleneck is the low-throughput nature of early-stage AED screens in conventional rodent models. This process could potentially be expedited by using simpler invertebrate systems, such as the nematode Caenorhabditis elegans.
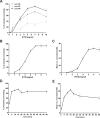
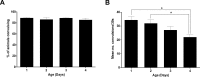
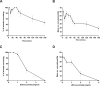
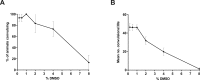
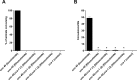
New method: Head-bobbing convulsions were previously reported to be inducible by pentylenetetrazol (PTZ) in C. elegans with loss-of-function mutations in unc-49, which encodes a GABAA receptor. Given that epilepsy-linked mutations in human GABAA receptors are well documented, this could represent a clinically-relevant system for early-stage AED screens. However, the original agar plate-based assay is unsuited to large-scale screening and has not been validated for identifying AEDs. Therefore, we established an alternative streamlined, higher-throughput approach whereby mutants were treated with PTZ and AEDs via liquid-based incubation.
Results: Convulsions induced within minutes of PTZ exposure in unc-49 mutants were strongly inhibited by the established AED ethosuximide. This protective activity was independent of ethosuximide's suggested target, the T-type calcium channel, as a null mutation in the worm cca-1 ortholog did not affect ethosuximide's anticonvulsant action.
Comparison with existing method: Our streamlined assay is AED-validated, feasible for higher throughput compound screens, and can facilitate insights into AED mechanisms of action.
Conclusions: Based on an epilepsy-associated genetic background, this C. elegans unc-49 model of seizure-like activity presents an ethical, higher throughput alternative to conventional rodent seizure models for initial AED screens.
Keywords: Anticonvulsant; Caenorhabditis elegans; Calcium channel; Drug screens; Epilepsy; Ethosuximide; GABA receptor; Pentylenetetrazol.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
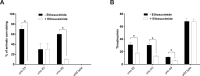
References
-
- Audenaert D., Schwartz E., Claeys K.G., Claes L., Deprez L., Suls A., Van Dyck T., Lagae L., Van Broeckhoven C., Macdonald R.L., De Jonghe P. A novel GABRG2 mutation associated with febrile seizures. Neurology. 2006;67:687–690. - PubMed
-
- Baines R.A., Giachello C.N.G., Lin W.-H. Chapter 24 - Drosophila. In: Pitkänen A., Buckmaster P.S., Galanopoulou A.S., Moshé S.L., editors. Models of Seizures and Epilepsy (Second Edition) Academic Press; 2017. pp. 345–358.
-
- Bargmann C.I. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

