Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase
- PMID: 30189596
- PMCID: PMC6225388
- DOI: 10.3390/molecules23092260
Hypoglycemic Efficacy of Docking Selected Natural Compounds against α-Glucosidase and α-Amylase
Abstract
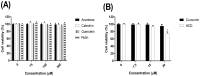
The inhibition of α-glucosidase and α-amylase is a clinical strategy for the treatment of type II diabetes, and herbal medicines have been reported to credibly alleviate hyperglycemia. Our previous study has reported some constituents from plant or herbal sources targeted to α-glucosidase and α-amylase via molecular docking and enzymatic measurement, but the hypoglycemic potencies in cell system and mice have not been validated yet. This study was aimed to elucidate the hypoglycemic efficacy of docking selected compounds in cell assay and oral glucose and starch tolerance tests of mice. All test compounds showed the inhibition of α-glucosidase activity in Caco-2 cells. The decrease of blood sugar levels of test compounds in 30 min and 60 min of mice after OGTT and OSTT, respectively and the decreased glucose levels of test compounds were significantly varied in acarbose. Taken altogether, in vitro and in vivo experiments suggest that selected natural compounds (curcumin, antroquinonol, HCD, docosanol, tetracosanol, rutin, and actinodaphnine) via molecular docking were confirmed as potential candidates of α-glucosidase and α-amylase inhibitors for treating diabetes.
Keywords: natural compound; α-amylase; α-glucosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- World Health Organization . Global Status Report on Noncommunicable Diseases 2014. WHO Press; Geneva, Switzerland: 2014. pp. 1–302.
-
- Inzucchi S.E., Bergenstal R.M., Buse J.B., Diamant M., Ferrannini E., Nauck M., Peters A.L., Tsapas A., Wender R., Matthews D.R. Management of hyperglycaemia in type 2 diabetes: A Patient-Centered Approach. Position Statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2012;55:1577–1596. doi: 10.1007/s00125-012-2534-0. - DOI - PubMed
-
- Holman R.R., Coleman R.L., Chan J.C.N., Chiasson J.-L., Feng H., Ge J., Gerstein H.C., Gray R., Huo Y., Lang Z., et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Diabetes Endocrinol. 2017;5:877–886. doi: 10.1016/S2213-8587(17)30309-1. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

