An Empirical Analysis of the Perceived Challenges and Benefits of Introducing Biosimilars in Bangladesh: A Paradigm Shift
- PMID: 30189610
- PMCID: PMC6163433
- DOI: 10.3390/biom8030089
An Empirical Analysis of the Perceived Challenges and Benefits of Introducing Biosimilars in Bangladesh: A Paradigm Shift
Abstract
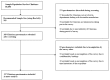
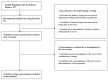
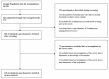
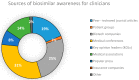
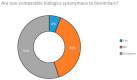
The high demand for and resulting financial success of biopharmaceutical products over the last three decades have seen the door open for close copies of these biological products, also known as biosimilars. This paper seeks to collate all relevant published intelligence with acquired survey data to assess the weight of available evidence that these products hold immense potential for the pharmaceutical industry in terms of their applications and benefits. Biosimilars also pose to be of great promise to the Bangladesh pharmaceutical industry, with the commitment of drastically reducing its dependence on foreign imports of biopharmaceutics to meet local demand. Our questionnaire based survey involved 100 Clinicians, 50 Industry Experts and 100 Academicians. The study found that majority of Industry Experts (72%) and Academicians (63%) shared a different concept of biosimilars opposed to majority of Clinicians (78%). Majority of Academicians (68%) and Industry Experts (61%) also shared a different belief from that of most Clinicians (61%) regarding the need for updating the existing regulatory guidelines. The study also showed that Clinicians (67%), Industry Experts (83%) and Academicians (80%) highlighted the benefit of lower costs of biosimilars. Furthermore, the quality data obtained from the survey results allowed us to evaluate and provide recommendations for stakeholders on the need for increased biosimilar awareness, pharmacovigilance and safety in Bangladesh.
Keywords: Bangladesh pharmaceutical industry; biologics; biosimilars; interchangeability.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
The Breakthrough of Biosimilars: A Twist in the Narrative of Biological Therapy.Biomolecules. 2019 Aug 24;9(9):410. doi: 10.3390/biom9090410. Biomolecules. 2019. PMID: 31450637 Free PMC article. Review.
-
Safety of Biologics, Including Biosimilars: Perspectives on Current Status and Future Direction.Drug Saf. 2018 Nov;41(11):1013-1022. doi: 10.1007/s40264-018-0684-9. Drug Saf. 2018. PMID: 29796832 Review.
-
Risk-based Process Development of Biosimilars as Part of the Quality by Design Paradigm.PDA J Pharm Sci Technol. 2013 Nov-Dec;67(6):569-80. doi: 10.5731/pdajpst.2013.00943. PDA J Pharm Sci Technol. 2013. PMID: 24265299
-
PDA Biosimilars Workshop Report (September 27-28, 2018)-Getting It Right the First Time for Biosimilar Marketing Applications.PDA J Pharm Sci Technol. 2019 Jul-Aug;73(4):401-416. doi: 10.5731/pdajpst.2019.010215. Epub 2019 Apr 19. PDA J Pharm Sci Technol. 2019. PMID: 31004040
-
Pharmacovigilance and biosimilars: considerations, needs and challenges.Expert Opin Biol Ther. 2013 Jul;13(7):1039-47. doi: 10.1517/14712598.2013.783560. Epub 2013 Mar 26. Expert Opin Biol Ther. 2013. PMID: 23527621 Review.
Cited by
-
Barriers and Enablers Affecting the Uptake of Biosimilar Medicines Viewed Through the Lens of Actor Network Theory: A Systematic Review.BioDrugs. 2024 Jul;38(4):541-555. doi: 10.1007/s40259-024-00659-0. Epub 2024 Jun 15. BioDrugs. 2024. PMID: 38879730 Free PMC article.
-
The Breakthrough of Biosimilars: A Twist in the Narrative of Biological Therapy.Biomolecules. 2019 Aug 24;9(9):410. doi: 10.3390/biom9090410. Biomolecules. 2019. PMID: 31450637 Free PMC article. Review.
-
Development and performance evaluation of a rapid in-house ELISA for retrospective serosurveillance of SARS-CoV-2.PLoS One. 2021 Feb 2;16(2):e0246346. doi: 10.1371/journal.pone.0246346. eCollection 2021. PLoS One. 2021. PMID: 33529223 Free PMC article.
-
Biosimilars Targeting Pathogens: A Comprehensive Review of Their Role in Bacterial, Fungal, Parasitic, and Viral Infections.Pharmaceutics. 2025 Apr 28;17(5):581. doi: 10.3390/pharmaceutics17050581. Pharmaceutics. 2025. PMID: 40430873 Free PMC article. Review.
-
Clinical and Regulatory Concerns of Biosimilars: A Review of Literature.Int J Environ Res Public Health. 2020 Aug 11;17(16):5800. doi: 10.3390/ijerph17165800. Int J Environ Res Public Health. 2020. PMID: 32796549 Free PMC article. Review.
References
-
- Chopra I., Arkells N., Chopra A. Biosimilars: Barriers and Opportunities to Market Access in the United States. Value Health. 2018;21:S93. doi: 10.1016/j.jval.2018.04.625. - DOI
-
- WHO Guidelines on Evaluation of Similar Biotherapeutic Products (SBPs) [(accessed on 17 April 2017)];2009 Retrieved 17 April 2017. Available online: http://apps.who.int/medicinedocs/en/d/Js19941en.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

