Insonation of Systemically Delivered Cisplatin-Loaded Microbubbles Significantly Attenuates Nephrotoxicity of Chemotherapy in Experimental Models of Head and Neck Cancer
- PMID: 30189620
- PMCID: PMC6162676
- DOI: 10.3390/cancers10090311
Insonation of Systemically Delivered Cisplatin-Loaded Microbubbles Significantly Attenuates Nephrotoxicity of Chemotherapy in Experimental Models of Head and Neck Cancer
Abstract
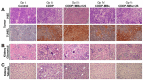
The use of cisplatin (CDDP), the most common chemotherapy drug for head and neck cancer, is limited by its undesirable side effects, especially nephrotoxicity. We investigated ultrasound microbubbles (USMB) as a tool to increase the local intra-tumoral CDDP level while decreasing systemic CDDP cytotoxicity. We allowed CDDP to interact with human serum albumin and then sonicated the resulting CDDP‒albumin complex to generate CDDP-loaded MBs (CDDP-MBs). We then established a head-and-neck tumor-bearing mouse model by implanting FaDu-fLuc/GFP cells into severe combined immunodeficiency mice and used IVIS® bioluminescence imaging to determine the tumor xenograft formation and size. Twice weekly (until Day 33), we administered CDDP only, CDDP + MBs + US, CDDP-MBs, or CDDP-MBs + US intravenously by tail-vein injection. The US treatment was administered at the tumor site immediately after injection. The in vivo systemic distribution of CDDP indicated that the kidney was the most vulnerable organ, followed by the liver, and then the inner ear. However, CDDP uptake into the kidney and liver was significantly decreased in both the CDDP-MBs and CDDP-MBs + US groups, suggesting that MB binding significantly reduced the systemic toxicity of CDDP. The CDDP-MBs + US treatment reduced the tumor size as effectively as conventional CDDP-only chemotherapy. Therefore, the combination of CDDP-MBs with ultrasound is effective and significantly attenuates CDDP-associated nephrotoxicity, indicating a promising clinical potential for this approach.
Keywords: chemotherapy; cisplatin; head and neck cancer; microbubble; nephrotoxicity; ultrasound.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
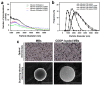
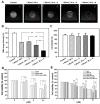
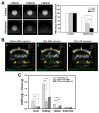
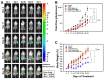



References
-
- Sasaki N., Ishi K., Kudo N., Nakayama S.M.M., Nakamura K., Morishita K., Ohta H., Ishizuka M., Takiguchi M. Spatial and temporal profile of cisplatin delivery by ultrasound-assisted intravesical chemotherapy in a bladder cancer model. PLoS ONE. 2017;12:e0188093. doi: 10.1371/journal.pone.0188093. - DOI - PMC - PubMed
Grants and funding
- MOST104-2314-B-016-032-MY3/Ministry of Science and Technology, Taiwan
- TSGH-C107-009/Tri-Service General Hospital
- MAB-107-001/Medical Affairs Bureau Ministry of National Defense
- MOST106-2221-E-011-043-MY3/Ministry of Science and Technology, Taiwan
- NTUST-TSGH-107-02/National Taiwan University of Science and Technology
- MOST103-2314-B-016-003/Ministry of Science and Technology, Taiwan
- TSGH-C104-043/Tri-Service General Hospital
- MAB107-002/Medical Affairs Bureau Ministry of National Defense
- A1071025/Teh-Tzer Study Group for Human Medical Research Foundation
- A1041001/Teh-Tzer Study Group for HumanMedical Research Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

