Histone Deacetylase Inhibitors and Diabetic Kidney Disease
- PMID: 30189630
- PMCID: PMC6165182
- DOI: 10.3390/ijms19092630
Histone Deacetylase Inhibitors and Diabetic Kidney Disease
Abstract
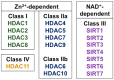
Despite recent clinical trial advances and improvements in clinical care, kidney disease due to diabetes remains the most common cause of chronic kidney failure worldwide. In the search for new treatments, recent attentions have turned to drug repurposing opportunities, including study of the histone deacetylase (HDAC) inhibitor class of agents. HDACs are a group of enzymes that remove functional acetyl groups from histone and non-histone proteins and they can affect cellular function through both epigenetic and non-epigenetic means. Over the past decade, several HDAC inhibitors have been adopted into clinical practice, primarily for the treatment of hematological malignancy, whereas other existing therapies (for instance valproate) have been found to have HDAC inhibitory effects. Here we review the current HDAC inhibitors in the clinic and under development; the literature evidence supporting the renoprotective effects of HDAC inhibitors in experimental diabetic kidney disease; and the adverse effect profiles that may prevent existing therapies from entering the clinic for this indication. Whereas recent research efforts have shed light on the fundamental actions of HDACs in the diabetic kidney, whether these efforts will translate into novel therapies for patients will require more specific and better-tolerated therapies.
Keywords: acetylation; diabetes; epigenetics; histone; kidney disease; nephropathy.
Conflict of interest statement
A.A. has received research support through his institution from Boehringer Ingelheim and AstraZeneca and is listed as an inventor on an unrelated patent application filed by Boehringer Ingelheim.
Figures

References
-
- Advani A., Huang Q., Thai K., Advani S.L., White K.E., Kelly D.J., Yuen D.A., Connelly K.A., Marsden P.A., Gilbert R.E. Long-term administration of the histone deacetylase inhibitor vorinostat attenuates renal injury in experimental diabetes through an endothelial nitric oxide synthase-dependent mechanism. Am. J. Pathol. 2011;178:2205–2214. doi: 10.1016/j.ajpath.2011.01.044. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

