Involvement of Up-Regulation of DR5 Expression and Down-Regulation of c-FLIP in Niclosamide-Mediated TRAIL Sensitization in Human Renal Carcinoma Caki Cells
- PMID: 30189637
- PMCID: PMC6225471
- DOI: 10.3390/molecules23092264
Involvement of Up-Regulation of DR5 Expression and Down-Regulation of c-FLIP in Niclosamide-Mediated TRAIL Sensitization in Human Renal Carcinoma Caki Cells
Abstract
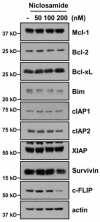
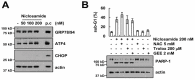
Niclosamide is used to treat intestinal parasite infections, as being an anthelmintic drug. Recently, several papers suggest the niclosamide inhibits multiple signaling pathways, which are highly activated and mutated in cancer. Here, niclosamide was evaluated for identifying strategies to overcome tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) resistance. Although niclosamide (100⁻200 nM) alone did not bring about cell death, combinations of niclosamide and TRAIL led to apoptotic cell death in carcinoma cells, but not in normal cells. Niclosamide markedly increased DR5 protein levels, including cell-surface DR5, and decreased c-FLIP protein levels. Down-regulation of DR5 by specific small interfering RNA (siRNA) and ectopic expression of c-FLIP markedly blocked niclosamide plus TRAIL-induced apoptosis. Our findings provide that niclosamide could overcome resistance to TRAIL through up-regulating DR5 on the cell surface and down-regulating c-FLIP in cancer cells. Taken together, niclosamide may be an attractive candidate to overcome TRAIL resistance.
Keywords: DR5; TRAIL; apoptosis; c-FLIP; niclosamide.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Rosiglitazone promotes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP.Free Radic Biol Med. 2008 Mar 15;44(6):1055-68. doi: 10.1016/j.freeradbiomed.2007.12.001. Epub 2007 Dec 8. Free Radic Biol Med. 2008. PMID: 18164688
-
Carnosic acid sensitized TRAIL-mediated apoptosis through down-regulation of c-FLIP and Bcl-2 expression at the post translational levels and CHOP-dependent up-regulation of DR5, Bim, and PUMA expression in human carcinoma caki cells.Oncotarget. 2015 Jan 30;6(3):1556-68. doi: 10.18632/oncotarget.2727. Oncotarget. 2015. PMID: 25596735 Free PMC article.
-
6-Shogaol enhances renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated cytochrome c release and down-regulation of c-FLIP(L) expression.Chem Biol Interact. 2015 Feb 25;228:69-78. doi: 10.1016/j.cbi.2015.01.020. Epub 2015 Jan 22. Chem Biol Interact. 2015. PMID: 25619640
-
Glucocorticoid receptor antagonist sensitizes TRAIL-induced apoptosis in renal carcinoma cells through up-regulation of DR5 and down-regulation of c-FLIP(L) and Bcl-2.J Mol Med (Berl). 2012 Mar;90(3):309-19. doi: 10.1007/s00109-011-0821-8. Epub 2011 Oct 19. J Mol Med (Berl). 2012. PMID: 22008998
-
Combination of niclosamide and current therapies to overcome resistance for cancer: New frontiers for an old drug.Biomed Pharmacother. 2022 Nov;155:113789. doi: 10.1016/j.biopha.2022.113789. Epub 2022 Oct 8. Biomed Pharmacother. 2022. PMID: 36271567 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

