Nucleus- and plastid-targeted annexin 5 promotes reproductive development in Arabidopsis and is essential for pollen and embryo formation
- PMID: 30189843
- PMCID: PMC6127919
- DOI: 10.1186/s12870-018-1405-3
Nucleus- and plastid-targeted annexin 5 promotes reproductive development in Arabidopsis and is essential for pollen and embryo formation
Abstract
Background: Pollen development is a strictly controlled post-meiotic process during which microspores differentiate into microgametophytes and profound structural and functional changes occur in organelles. Annexin 5 is a calcium- and lipid-binding protein that is highly expressed in pollen grains and regulates pollen development and physiology. To gain further insights into the role of ANN5 in Arabidopsis development, we performed detailed phenotypic characterization of Arabidopsis plants with modified ANN5 levels. In addition, interaction partners and subcellular localization of ANN5 were analyzed to investigate potential functions of ANN5 at cellular level.
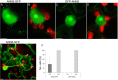
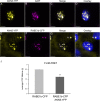
Results: Here, we report that RNAi-mediated suppression of ANN5 results in formation of smaller pollen grains, enhanced pollen lethality, and delayed pollen tube growth. ANN5 RNAi knockdown plants also displayed aberrant development during the transition from the vegetative to generative phase and during embryogenesis, reflected by delayed bolting time and reduced embryo size, respectively. At the subcellular level, ANN5 was delivered to the nucleus, nucleolus, and cytoplasm, and was frequently localized in plastid nucleoids, suggesting a likely role in interorganellar communication. Furthermore, ANN5-YFP co-immunoprecipitated with RABE1b, a putative GTPase, and interaction in planta was confirmed in plastidial nucleoids using FLIM-FRET analysis.
Conclusions: Our findings let us to propose that ANN5 influences basal cell homeostasis via modulation of plastid activity during pollen maturation. We hypothesize that the role of ANN5 is to orchestrate the plastidial and nuclear genome activities via protein-protein interactions however not only in maturing pollen but also during the transition from the vegetative to the generative growth and seed development.
Keywords: Accession; Annexin; Arabidopsis; Chlorophyll; Embryo; Nucleoid; Plastid; Pollen grain; Rab GTPase; Seed.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Annexin5 plays a vital role in Arabidopsis pollen development via Ca2+-dependent membrane trafficking.PLoS One. 2014 Jul 14;9(7):e102407. doi: 10.1371/journal.pone.0102407. eCollection 2014. PLoS One. 2014. PMID: 25019283 Free PMC article.
-
Arabidopsis annexin 5 is involved in maintenance of pollen membrane integrity and permeability.J Exp Bot. 2022 Jan 5;73(1):94-109. doi: 10.1093/jxb/erab419. J Exp Bot. 2022. PMID: 34522949
-
The ARID-HMG DNA-binding protein AtHMGB15 is required for pollen tube growth in Arabidopsis thaliana.Plant J. 2014 Sep;79(5):741-56. doi: 10.1111/tpj.12582. Epub 2014 Jul 23. Plant J. 2014. PMID: 24923357
-
Cytoplasmic connection of sperm cells to the pollen vegetative cell nucleus: potential roles of the male germ unit revisited.J Exp Bot. 2011 Mar;62(5):1621-31. doi: 10.1093/jxb/err032. J Exp Bot. 2011. PMID: 21357775 Review.
-
New insights into plastid nucleoid structure and functionality.Planta. 2013 Mar;237(3):653-64. doi: 10.1007/s00425-012-1817-5. Epub 2012 Dec 5. Planta. 2013. PMID: 23212213 Review.
Cited by
-
Secretory Vesicles Targeted to Plasma Membrane During Pollen Germination and Tube Growth.Front Cell Dev Biol. 2021 Jan 21;8:615447. doi: 10.3389/fcell.2020.615447. eCollection 2020. Front Cell Dev Biol. 2021. PMID: 33553150 Free PMC article. Review.
-
Genome-Wide Characterization and Abiotic Stresses Expression Analysis of Annexin Family Genes in Poplar.Int J Mol Sci. 2022 Jan 3;23(1):515. doi: 10.3390/ijms23010515. Int J Mol Sci. 2022. PMID: 35008941 Free PMC article.
-
Delineating Calcium Signaling Machinery in Plants: Tapping the Potential through Functional Genomics.Curr Genomics. 2021 Dec 30;22(6):404-439. doi: 10.2174/1389202922666211130143328. Curr Genomics. 2021. PMID: 35340362 Free PMC article. Review.
-
Comprehensive analyses of the annexin (ANN) gene family in Brassica rapa, Brassica oleracea and Brassica napus reveals their roles in stress response.Sci Rep. 2020 Mar 9;10(1):4295. doi: 10.1038/s41598-020-59953-w. Sci Rep. 2020. PMID: 32152363 Free PMC article.
-
Genome-Wide Identification of the GhANN Gene Family and Functional Validation of GhANN11 and GhANN4 under Abiotic Stress.Int J Mol Sci. 2024 Feb 4;25(3):1877. doi: 10.3390/ijms25031877. Int J Mol Sci. 2024. PMID: 38339155 Free PMC article.
References
-
- Owen HA, Makaroff CA. Ultrastructure of microsporogenesis and microgametogenesis in Arabidopsis thaliana (L.) Heynh. Ecotype Wassilewskija (Brassicaceae) Protoplasma. 1995;185:7–21. doi: 10.1007/BF01272749. - DOI
-
- Franchi G, Bellani L, Nepi M, Pacini E. Types of carbohydrate reserves in pollen: localization, systematic distribution and ecophysiological significance. Flora. 1996;191:143–159. doi: 10.1016/S0367-2530(17)30706-5. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

