Macrophages: friend or foe in idiopathic pulmonary fibrosis?
- PMID: 30189872
- PMCID: PMC6127991
- DOI: 10.1186/s12931-018-0864-2
Macrophages: friend or foe in idiopathic pulmonary fibrosis?
Abstract
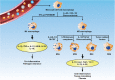
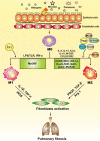
Idiopathic pulmonary fibrosis (IPF) is a prototype of lethal, chronic, progressive interstitial lung disease of unknown etiology. Over the past decade, macrophage has been recognized to play a significant role in IPF pathogenesis. Depending on the local microenvironments, macrophages can be polarized to either classically activated (M1) or alternatively activated (M2) phenotypes. In general, M1 macrophages are responsible for wound healing after alveolar epithelial injury, while M2 macrophages are designated to resolve wound healing processes or terminate inflammatory responses in the lung. IPF is a pathological consequence resulted from altered wound healing in response to persistent lung injury. In this review, we intend to summarize the current state of knowledge regarding the process of macrophage polarization and its mediators in the pathogenesis of pulmonary fibrosis. Our goal is to update the understanding of the mechanisms underlying the initiation and progression of IPF, and by which, we expect to provide help for developing effective therapeutic strategies in clinical settings.
Keywords: Alternatively activated (M2) macrophages; Classically activated (M1) macrophages; IPF; Macrophage polarization; Therapeutic strategies.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

