Characterization of a serine protease inhibitor from Trichinella spiralis and its participation in larval invasion of host's intestinal epithelial cells
- PMID: 30189888
- PMCID: PMC6127903
- DOI: 10.1186/s13071-018-3074-3
Characterization of a serine protease inhibitor from Trichinella spiralis and its participation in larval invasion of host's intestinal epithelial cells
Abstract
Background: Trichinella spiralis serine protease inhibitor (TsSPI) was identified in ES proteins of adult worms (AW), the TsSPI gene was highly expressed at enteral stage worms (AW and newborn larvae), distributed mainly in the cuticle and stichosome of this nematode. Vaccination of mice with rTsSPI exhibited a 62.2% reduction of intestinal AW and a 57.25% reduction of muscle larvae after larval challenge. The aim of this study was to investigate the biological characteristics of TsSPI and its roles in the process of T. spiralis invasion of host's intestinal epithelium cells (IECs).
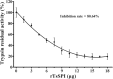
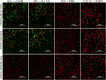
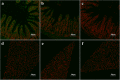
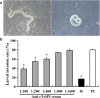
Methods: The rTsSPI inhibition on trypsin enzymatic activity was detected by SDS-PAGE and spectrophotometry. The binding of rTsSPI with intestinal epithelium from normal mice and the primary cultured mouse intestinal epithelium cells (IECs) was examined by indirect immunofluorescent (IIF), the cellular localization of rTsSPI binding to IECs was observed by confocal microscopy. The inhibition of anti-rTsSPI serum on T. spiralis invasion of IECs was determined by an in vitro invasion assay. Anti-rTsSPI antibody cytotoxicity on the newborn larvae (NBL) was also determined.
Results: The rTsSPI had the inhibitory activity against porcine trypsin. The rTsSPI specifically bound to the intestinal epithelium from normal mice and primary cultured mouse IECs, and the binding sites were located in IEC membrane and cytoplasm. Anti-rTsSPI antibodies depressed the larval invasion of IECs with a dose-dependent mode. Anti-rTsSPI antibodies also participated in the destruction of T. spiralis NBL via an ADCC-mediated manner.
Conclusions: TsSPI might participate in the T. spiralis larval invasion of IECs and it is likely the potential vaccine target against T. spiralis enteral stages.
Keywords: Inhibitory activity; Larval invasion; Serine protease inhibitor; Trichinella spiralis; Trypsin.
Conflict of interest statement
Ethics approval
All the animal experiments were authorized by the Zhengzhou University Life Science Ethics Committee (No. SCXK 2015-0005).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures





Similar articles
-
In vitro silencing of a serine protease inhibitor suppresses Trichinella spiralis invasion, development, and fecundity.Parasitol Res. 2019 Jul;118(7):2247-2255. doi: 10.1007/s00436-019-06344-4. Epub 2019 May 12. Parasitol Res. 2019. PMID: 31081529
-
Molecular characterization of a 31 kDa protein from Trichinella spiralis and its induced immune protection in BALB/c mice.Parasit Vectors. 2018 Dec 5;11(1):625. doi: 10.1186/s13071-018-3198-5. Parasit Vectors. 2018. PMID: 30518426 Free PMC article.
-
RNAi-mediated silencing of Trichinella spiralis serpin-type serine protease inhibitors results in a reduction in larval infectivity.Vet Res. 2020 Nov 23;51(1):139. doi: 10.1186/s13567-020-00860-3. Vet Res. 2020. PMID: 33225967 Free PMC article.
-
Vaccines against Trichinella spiralis: Progress, challenges and future prospects.Transbound Emerg Dis. 2018 Dec;65(6):1447-1458. doi: 10.1111/tbed.12917. Epub 2018 Jun 6. Transbound Emerg Dis. 2018. PMID: 29873198 Review.
-
A pivotal role for glycans at the interface between Trichinella spiralis and its host.Vet Parasitol. 2001 Nov 22;101(3-4):249-60. doi: 10.1016/s0304-4017(01)00570-2. Vet Parasitol. 2001. PMID: 11707300 Review.
Cited by
-
In vitro silencing of a serine protease inhibitor suppresses Trichinella spiralis invasion, development, and fecundity.Parasitol Res. 2019 Jul;118(7):2247-2255. doi: 10.1007/s00436-019-06344-4. Epub 2019 May 12. Parasitol Res. 2019. PMID: 31081529
-
Adjuvanticity of β -Glucan for Vaccine Against Trichinella spiralis.Front Cell Dev Biol. 2021 Jul 12;9:701708. doi: 10.3389/fcell.2021.701708. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34322488 Free PMC article.
-
Proteases secreted by Trichinella spiralis intestinal infective larvae damage the junctions of the intestinal epithelial cell monolayer and mediate larval invasion.Vet Res. 2022 Mar 7;53(1):19. doi: 10.1186/s13567-022-01032-1. Vet Res. 2022. PMID: 35255974 Free PMC article.
-
Binding of Trichinella spiralis C-type lectin with syndecan-1 on intestinal epithelial cells mediates larval invasion of intestinal epithelium.Vet Res. 2023 Oct 2;54(1):86. doi: 10.1186/s13567-023-01217-2. Vet Res. 2023. PMID: 37784173 Free PMC article.
-
Effects of Trichinella spiralis and its serine protease inhibitors on autophagy of host small intestinal cells.Infect Immun. 2023 Nov 16;91(11):e0010323. doi: 10.1128/iai.00103-23. Epub 2023 Oct 24. Infect Immun. 2023. PMID: 37874164 Free PMC article.
References
-
- Cui J, Wang ZQ, Xu BL. The epidemiology of human trichinellosis in China during 2004–2009. Acta Trop. 2011;118:1–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

