Systematic Review on the Utilization of Maintenance Intravesical Chemotherapy in the Management of Non-muscle-invasive Bladder Cancer
- PMID: 30190111
- PMCID: PMC7810510
- DOI: 10.1016/j.euf.2018.08.019
Systematic Review on the Utilization of Maintenance Intravesical Chemotherapy in the Management of Non-muscle-invasive Bladder Cancer
Abstract
Context: Current guidelines remain ill-defined regarding the optimal intravesical chemotherapy type and regimen for the treatment of non-muscle-invasive bladder cancer (NMIBC). Although maintenance therapy is a standard part of bacillus Calmette-Guerin (BCG) therapy, its role in the context of chemotherapy remains debatable.
Objective: We reviewed the literature regarding the utilization of intravesical maintenance chemotherapy in the treatment of NMIBC to determine its impact on recurrence, progression, and survival.
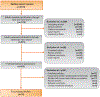
Evidence acquisition: A systematic search was conducted using Ovid and Medline according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines to identify studies between 1970 and 2018 reporting on the utilization of maintenance intravesical chemotherapy. Only randomized controlled trials (RCTs) that included a comparison between an induction regimen and an induction plus maintenance regimen were included.
Evidence synthesis: Sixteen RCTs were included in the final analysis. The most commonly studied intravesical chemotherapy agents used in maintenance regimens were epirubicin, doxorubicin, and mitomycin C. Several maintenance schedules were utilized, some as short as 3mo and others as long as 3 yr, while the most common maintenance regimen utilized was monthly instillation for 1 yr. Of the 16 trials, 13 reported no significant improvement in recurrence for patients receiving maintenance compared with no maintenance, and none of the trials demonstrated a significant impact on progression or survival.
Conclusions: Intermediate length maintenance regimens lasting 7-12mo were the most common maintenance regimens utilized. There was considerable heterogeneity between trial design and duration of follow-up, making direct comparisons for recurrence, progression, and survival outcomes between trials challenging. Although maintenance intravesical chemotherapy is suggested as a treatment option for patients with NMIBC by some guidelines, the majority of evidence suggested that it provided no significant advantage over induction therapy alone with respect to recurrence, progression, or survival.
Patient summary: In this review, we reviewed prior clinical trials to determine whether prolonged intravesical chemotherapy ("maintenance therapy") improved the rates of recurrence, progression, and survival. Where differences were found in favor of maintenance therapy, there was no statistical significance demonstrated, possibly due to the underpowered nature of the study design. While there was no consensus on an optimal agent or maintenance schedule, we found no evidence to suggest that maintenance therapy would improve recurrence, progression, or survival.
Keywords: Bladder cancer; Chemotherapy; Intravesical; Maintenance.
Copyright © 2018 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
References
-
- Kamat AM, Hahn NM, Efstathiou JA, Lerner SP, Malmstrom PU, Choi W, et al. Bladder cancer. Lancet. 2016;388:2796–810. - PubMed
-
- Chang SS, Boorjian SA, Chou R, Clark PE, Daneshmand S, Konety BR, et al. Diagnosis and Treatment of Non-Muscle Invasive Bladder Cancer: AUA/SUO Guideline. J Urol. 2016;196:1021–9. - PubMed
-
- Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71:447–61. - PubMed
-
- Nielsen ME, Smith AB, Pruthi RS, Guzzo TJ, Amiel G, Shore N, et al. Reported use of intravesical therapy for non-muscle-invasive bladder cancer (NMIBC): results from the Bladder Cancer Advocacy Network (BCAN) survey. BJU Int. 2012;110:967–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical