Remote ischemic post-conditioning promotes hematoma resolution via AMPK-dependent immune regulation
- PMID: 30190288
- PMCID: PMC6170180
- DOI: 10.1084/jem.20171905
Remote ischemic post-conditioning promotes hematoma resolution via AMPK-dependent immune regulation
Abstract
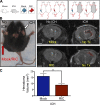
Spontaneous intracerebral hemorrhage (ICH) produces the highest acute mortality and worst outcomes of all stroke subtypes. Hematoma volume is an independent determinant of ICH patient outcomes, making clot resolution a primary goal of clinical management. Herein, remote-limb ischemic post-conditioning (RIC), the repetitive inflation-deflation of a blood pressure cuff on a limb, accelerated hematoma resolution and improved neurological outcomes after ICH in mice. Parabiosis studies revealed RIC accelerated clot resolution via a humoral-mediated mechanism. Whereas RIC increased anti-inflammatory macrophage activation, myeloid cell depletion eliminated the beneficial effects of RIC after ICH. Myeloid-specific inactivation of the metabolic regulator, AMPKα1, attenuated RIC-induced anti-inflammatory macrophage polarization and delayed hematoma resolution, providing a molecular link between RIC and immune activation. Finally, chimera studies implicated myeloid CD36 expression in RIC-mediated neurological recovery after ICH. Thus, RIC, a clinically well-tolerated therapy, noninvasively modulates innate immune responses to improve ICH outcomes. Moreover, immunometabolic changes may provide pharmacodynamic blood biomarkers to clinically monitor the therapeutic efficacy of RIC.
© 2018 Vaibhav et al.
Figures








References
-
- Bhasin R.R., Xi G., Hua Y., Keep R.F., and Hoff J.T.. 2002. Experimental intracerebral hemorrhage: effect of lysed erythrocytes on brain edema and blood-brain barrier permeability. Acta Neurochir. Suppl. (Wien). 81:249–251. - PubMed
-
- Bøtker H.E., Kharbanda R., Schmidt M.R., Bøttcher M., Kaltoft A.K., Terkelsen C.J., Munk K., Andersen N.H., Hansen T.M., Trautner S., et al. 2010. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 375:727–734. 10.1016/S0140-6736(09)62001-8 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

