Clinical Pharmacology of Tisagenlecleucel in B-cell Acute Lymphoblastic Leukemia
- PMID: 30190371
- PMCID: PMC7433345
- DOI: 10.1158/1078-0432.CCR-18-0758
Clinical Pharmacology of Tisagenlecleucel in B-cell Acute Lymphoblastic Leukemia
Abstract
Purpose: Tisagenlecleucel is an anti-CD19 chimeric antigen receptor (CAR19) T-cell therapy approved for the treatment of children and young adults with relapsed/refractory (r/r) B-cell acute lymphoblastic leukemia (B-ALL).
Patients and methods: We evaluated the cellular kinetics of tisagenlecleucel, the effect of patient factors, humoral immunogenicity, and manufacturing attributes on its kinetics, and exposure-response analysis for efficacy, safety and pharmacodynamic endpoints in 79 patients across two studies in pediatric B-ALL (ELIANA and ENSIGN).
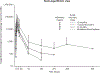
Results: Using quantitative polymerase chain reaction to quantify levels of tisagenlecleucel transgene, responders (N = 62) had ≈2-fold higher tisagenlecleucel expansion in peripheral blood than nonresponders (N = 8; 74% and 104% higher geometric mean Cmax and AUC0-28d, respectively) with persistence measurable beyond 2 years in responding patients. Cmax increased with occurrence and severity of cytokine release syndrome (CRS). Tisagenlecleucel continued to expand and persist following tocilizumab, used to manage CRS. Patients with B-cell recovery within 6 months had earlier loss of the transgene compared with patients with sustained clinical response. Clinical responses were seen across the entire dose range evaluated (patients ≤50 kg: 0.2 to 5.0 × 106/kg; patients >50 kg: 0.1 to 2.5 × 108 CAR-positive viable T cells) with no relationship between dose and safety. Neither preexisting nor treatment-induced antimurine CAR19 antibodies affected the persistence or clinical response.
Conclusions: Response to tisagenlecleucel was associated with increased expansion across a wide dose range. These results highlight the importance of cellular kinetics in understanding determinants of response to chimeric antigen receptor T-cell therapy.
©2018 American Association for Cancer Research.
Figures
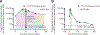

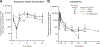

References
-
- Grupp SA, Laetsch TW, Buechner J, Bittencourt H, Maude SL, Verneris MR, et al. Analysis of a global registration trial of the efficacy and safety of CTL019 in pediatric and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL). Blood 2016;128:[abstract 221]. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

