Simultaneous polymerization and adhesion under hypoxia in sickle cell disease
- PMID: 30190429
- PMCID: PMC6156668
- DOI: 10.1073/pnas.1807405115
Simultaneous polymerization and adhesion under hypoxia in sickle cell disease
Abstract
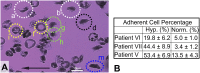
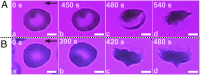
Polymerization and adhesion, dynamic processes that are hallmarks of sickle cell disease (SCD), have thus far been studied in vitro only separately. Here, we present quantitative results of the simultaneous and synergistic effects of adhesion and polymerization of deoxygenated sickle hemoglobin (HbS) in the human red blood cell (RBC) on the mechanisms underlying vasoocclusive pain crisis. For this purpose, we employ a specially developed hypoxic microfluidic platform, which is capable of inducing sickling and unsickling of RBCs in vitro, to test blood samples from eight patients with SCD. We supplemented these experimental results with detailed molecular-level computational simulations of cytoadherence and biorheology using dissipative particle dynamics. By recourse to image analysis techniques, we characterize sickle RBC maturation stages in the following order of the degree of adhesion susceptibility under hypoxia: sickle reticulocytes in circulation (SRs) → sickle mature erythrocytes (SMEs) → irreversibly sickled cells (ISCs). We show that (i) hypoxia significantly enhances sickle RBC adherence; (ii) HbS polymerization enhances sickle cell adherence in SRs and SMEs, but not in ISCs; (iii) SRs exhibit unique adhesion dynamics where HbS fiber projections growing outward from the cell surface create multiple sites of adhesion; and (iv) polymerization stimulates adhesion and vice versa, thereby establishing the bidirectional coupling between the two processes. These findings offer insights into possible mechanistic pathways leading to vasoocclusion crisis. They also elucidate the processes underlying the onset of occlusion that may involve circulating reticulocytes, which are more abundant in hemolytic anemias due to robust compensatory erythropoiesis.
Keywords: HbS polymerization; dissipative particle dynamics; hypoxia; microfluidics; sickle cell adhesion dynamics.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: D.P.P., S.Z.A., M.D., and S.S. have filed a patent based on the work presented in this paper.
Figures




Similar articles
-
Electrical Impedance Characterization of Erythrocyte Response to Cyclic Hypoxia in Sickle Cell Disease.ACS Sens. 2019 Jul 26;4(7):1783-1790. doi: 10.1021/acssensors.9b00263. Epub 2019 May 23. ACS Sens. 2019. PMID: 31083931 Free PMC article.
-
Quantifying Shear-Induced Deformation and Detachment of Individual Adherent Sickle Red Blood Cells.Biophys J. 2019 Jan 22;116(2):360-371. doi: 10.1016/j.bpj.2018.12.008. Epub 2018 Dec 18. Biophys J. 2019. PMID: 30612714 Free PMC article.
-
Mesoscopic Adaptive Resolution Scheme toward Understanding of Interactions between Sickle Cell Fibers.Biophys J. 2017 Jul 11;113(1):48-59. doi: 10.1016/j.bpj.2017.05.050. Biophys J. 2017. PMID: 28700924 Free PMC article.
-
Evolving treatment paradigms in sickle cell disease.Hematology Am Soc Hematol Educ Program. 2017 Dec 8;2017(1):440-446. doi: 10.1182/asheducation-2017.1.440. Hematology Am Soc Hematol Educ Program. 2017. PMID: 29222291 Free PMC article. Review.
-
Not all red cells sickle the same: Contributions of the reticulocyte to disease pathology in sickle cell anemia.Blood Rev. 2020 Mar;40:100637. doi: 10.1016/j.blre.2019.100637. Epub 2019 Nov 5. Blood Rev. 2020. PMID: 31735458 Free PMC article. Review.
Cited by
-
Elucidating parasite and host-cell factors enabling Babesia infection in sickle red cells under hypoxic/hyperoxic conditions.Blood Adv. 2023 Feb 28;7(4):649-663. doi: 10.1182/bloodadvances.2022008159. Blood Adv. 2023. PMID: 35977077 Free PMC article.
-
The Role of RBC Oxidative Stress in Sickle Cell Disease: From the Molecular Basis to Pathologic Implications.Antioxidants (Basel). 2021 Oct 13;10(10):1608. doi: 10.3390/antiox10101608. Antioxidants (Basel). 2021. PMID: 34679742 Free PMC article. Review.
-
Gene expression changes in sickle cell reticulocytes and their clinical associations.Sci Rep. 2023 Aug 8;13(1):12864. doi: 10.1038/s41598-023-40039-2. Sci Rep. 2023. PMID: 37553354 Free PMC article.
-
Synergistic Integration of Laboratory and Numerical Approaches in Studies of the Biomechanics of Diseased Red Blood Cells.Biosensors (Basel). 2018 Aug 10;8(3):76. doi: 10.3390/bios8030076. Biosensors (Basel). 2018. PMID: 30103419 Free PMC article. Review.
-
Microfluidics in Sickle Cell Disease Research: State of the Art and a Perspective Beyond the Flow Problem.Front Mol Biosci. 2021 Mar 8;7:558982. doi: 10.3389/fmolb.2020.558982. eCollection 2020. Front Mol Biosci. 2021. PMID: 33763448 Free PMC article. Review.
References
-
- Hebbel RP. Adhesion of sickle red cells to endothelium: Myths and future directions. Transfus Clin Biol. 2008;15:14–18. - PubMed
-
- Hebbel RP. Beyond hemoglobin polymerization: The red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214–237. - PubMed
-
- Hebbel RP, Boogaerts MAB, Eaton JW, Steinberg MH. Erythrocyte adherence to endothelium in sickle-cell anemia. A possible determinant of disease severity. N Engl J Med. 1980;302:992–995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

