Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders
- PMID: 30190436
- PMCID: PMC6156670
- DOI: 10.1073/pnas.1806501115
Mechanics of diseased red blood cells in human spleen and consequences for hereditary blood disorders
Abstract
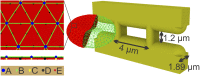
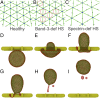
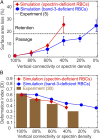
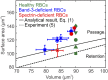
In red blood cell (RBC) diseases, the spleen contributes to anemia by clearing the damaged RBCs, but its unique ability to mechanically challenge RBCs also poses the risk of inducing other pathogenic effects. We have analyzed RBCs in hereditary spherocytosis (HS) and hereditary elliptocytosis (HE), two typical examples of blood disorders that result in membrane protein defects in RBCs. We use a two-component protein-scale RBC model to simulate the traversal of the interendothelial slit (IES) in the human spleen, a stringent biomechanical challenge on healthy and diseased RBCs that cannot be directly observed in vivo. In HS, our results confirm that the RBC loses surface due to weakened cohesion between the lipid bilayer and the cytoskeleton and reveal that surface loss may result from vesiculation of the RBC as it crosses IES. In HE, traversing IES induces sustained elongation of the RBC with impaired elasticity and fragmentation in severe disease. Our simulations thus suggest that in inherited RBC disorders, the spleen not only filters out pathological RBCs but also directly contributes to RBC alterations. These results provide a mechanistic rationale for different clinical outcomes documented following splenectomy in HS patients with spectrin-deficient and ankyrin-deficient RBCs and offer insights into the pathogenic role of human spleen in RBC diseases.
Keywords: cell fragmentation; hereditary elliptocytosis; hereditary spherocytosis; spleen; vesiculation.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. - PubMed
-
- Petroianu A. The Spleen. Bentham Science Publishers; Hilversum, The Netherlands: 2011.
-
- Buffet PA, et al. Ex vivo perfusion of human spleens maintains clearing and processing functions. Blood. 2006;107:3745–3752. - PubMed
-
- Safeukui I, et al. Retention of plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008;112:2520–2528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

