MEK inhibitors induce apoptosis via FoxO3a-dependent PUMA induction in colorectal cancer cells
- PMID: 30190455
- PMCID: PMC6127344
- DOI: 10.1038/s41389-018-0078-y
MEK inhibitors induce apoptosis via FoxO3a-dependent PUMA induction in colorectal cancer cells
Retraction in
-
Retraction Note: MEK inhibitors induce apoptosis via FoxO3a-dependent PUMA induction in colorectal cancer cells.Oncogenesis. 2024 Nov 21;13(1):41. doi: 10.1038/s41389-024-00543-0. Oncogenesis. 2024. PMID: 39572524 Free PMC article. No abstract available.
Abstract
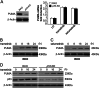
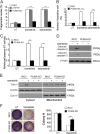
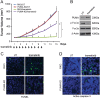
Mutations in BRAF are common to many cancers, including CRC. The MEK inhibitors are being investigated in BRAF-mutant CRC. In this study, we aimed to investigate how MEK inhibitor suppresses growth of BRAF-mutated CRC cells as well as its potential mechanisms. Our findings indicated that MEK inhibitor promote PUMA expression via ERK/FoxO3a signaling pathway. In addition, PUMA induction is essential for MEK inhibitor-induced apoptosis. Moreover, PUMA induction is required for MEK inhibitors to induced apoptosis in combination with cisplatin, dabrafenib, or Gefitinib. Knockdown of PUMA suppressed the anticancer effect of the MEK inhibitor in vivo. Our findings indicate a novel role for PUMA as a regulator of the antitumor effects of MEK inhibitor, suggesting that PUMA induction may modulate MEK inhibitor sensitivity.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

