The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis
- PMID: 30190486
- PMCID: PMC6127187
- DOI: 10.1038/s41598-018-30780-4
The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis
Erratum in
-
Publisher Correction: The diagnostic accuracy of circulating tumor DNA for the detection of EGFR-T790M mutation in NSCLC: a systematic review and meta-analysis.Sci Rep. 2018 Nov 19;8(1):17270. doi: 10.1038/s41598-018-35524-y. Sci Rep. 2018. PMID: 30451938 Free PMC article.
Abstract
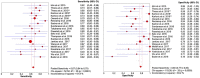
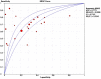
This pooled analysis aims at evaluating the diagnostic accuracy of circulating tumor (ct) DNA for the detection of EGFR-T790M mutation in NSCLC patients who progressed after EGFR-TKIs. Data from all published studies, reporting both sensitivity and specificity of plasma-based EGFR-T790M mutation testing by ctDNA were collected by searching in PubMed, Cochrane Library, American Society of Clinical Oncology, European Society of Medical Oncology and World Conference of Lung Cancer meeting proceedings. A total of twenty-one studies, with 1639 patients, were eligible. The pooled sensitivity of ctDNA analysis was 0.67 (95% CI: 0.64-0.70) and the pooled specificity was 0.80 (95% CI: 0.77-0.83). The pooled positive predictive value (PPV) was 0.85 (95% CI: 0.82-0.87) and the pooled negative predictive value (NPV) was 0.60 (95% CI: 0.56-0.63). The positive likelihood ratio (PLR) and negative likelihood ratio (NLR) were 2.67 (95% CI: 1.86-3.82) and 0.46 (95% CI: 0.38-0.54), respectively. The pooled diagnostic odds ratio (DOR) was 7.27 (4.39-12.05) and the area under the curve (AUC) of the summary receiver operating characteristics (sROC) curve was 0.77. The ctDNA analysis represents a promising, non-invasive approach to detect and monitor the T790M mutation status in NSCLC patients. Development of standardized methodologies and clinical validation are recommended.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. - DOI - PubMed
-
- Mitsudomi T, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. - DOI - PubMed
-
- Zhou C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

