The contribution of hormone sensitive lipase to adipose tissue lipolysis and its regulation by insulin in periparturient dairy cows
- PMID: 30190510
- PMCID: PMC6127149
- DOI: 10.1038/s41598-018-31582-4
The contribution of hormone sensitive lipase to adipose tissue lipolysis and its regulation by insulin in periparturient dairy cows
Abstract
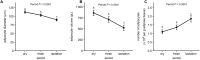
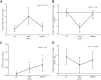
Hormone sensitive lipase (HSL) activation is part of the metabolic adaptations to the negative energy balance common to the mammalian periparturient period. This study determined HSL contribution to adipose tissue (AT) lipolysis and how insulin regulates its activity in periparturient dairy cows. Subcutaneous AT (SCAT) samples were collected at 11 d prepartum (dry) and 11 (fresh) and 24 d (lactation) postpartum. Basal and stimulated lipolysis (ISO) responses were determined using explant cultures. HSL contribution to lipolysis was assessed using an HSL inhibitor (CAY). Basal lipolysis was higher in SCAT at dry compared with fresh. CAY inhibited basal lipolysis negligibly at dry, but at fresh and lactation it reduced basal lipolysis by 36.1 ± 4.51% and 43.1 ± 4.83%, respectively. Insulin inhibited lipolysis more pronouncedly in dry compared to fresh. Results demonstrate that HSL contribution to basal lipolysis is negligible prepartum. However, HSL is a major driver of SCAT lipolytic responses postpartum. Lower basal lipolysis postpartum suggests that reduced lipogenesis is an important contributor to fatty acid release from SCAT. Loss of adipocyte sensitivity to the antilipolytic action of insulin develops in the early lactation period and supports a state of insulin resistance in AT of cows during the first month postpartum.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Hormone-sensitive lipase protein expression and extent of phosphorylation in subcutaneous and retroperitoneal adipose tissues in the periparturient dairy cow.J Dairy Sci. 2011 Sep;94(9):4514-23. doi: 10.3168/jds.2011-4145. J Dairy Sci. 2011. PMID: 21854923
-
Lipopolysaccharide induces lipolysis and insulin resistance in adipose tissue from dairy cows.J Dairy Sci. 2022 Jan;105(1):842-855. doi: 10.3168/jds.2021-20855. Epub 2021 Oct 23. J Dairy Sci. 2022. PMID: 34696909
-
Lipolysis modulates the biosynthesis of inflammatory lipid mediators derived from linoleic acid in adipose tissue of periparturient dairy cows.J Dairy Sci. 2020 Feb;103(2):1944-1955. doi: 10.3168/jds.2019-17256. Epub 2019 Nov 20. J Dairy Sci. 2020. PMID: 31759597
-
Molecular mechanisms regulating hormone-sensitive lipase and lipolysis.Biochem Soc Trans. 2003 Dec;31(Pt 6):1120-4. doi: 10.1042/bst0311120. Biochem Soc Trans. 2003. PMID: 14641008 Review.
-
Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease.J Mol Endocrinol. 2014 Jun;52(3):R199-222. doi: 10.1530/JME-13-0277. Epub 2014 Feb 27. J Mol Endocrinol. 2014. PMID: 24577718 Review.
Cited by
-
Identification of Plasma Fatty Acids in Four Lipid Classes to Understand Energy Metabolism at Different Levels of Ketonemia in Dairy Cows Using Thin Layer Chromatography and Gas Chromatographic Techniques (TLC-GC).Animals (Basel). 2020 Mar 29;10(4):571. doi: 10.3390/ani10040571. Animals (Basel). 2020. PMID: 32235301 Free PMC article.
-
Temporal patterns of liver and adipose lipase abundance across the periparturient period in multiparous Holstein dairy cows.JDS Commun. 2024 Sep 6;6(1):143-148. doi: 10.3168/jdsc.2024-0613. eCollection 2025 Jan. JDS Commun. 2024. PMID: 39877176 Free PMC article.
-
Harnessing the value of reproductive hormones in cattle production with considerations to animal welfare and human health.J Anim Sci. 2022 Jul 1;100(7):skac177. doi: 10.1093/jas/skac177. J Anim Sci. 2022. PMID: 35772763 Free PMC article. Review.
-
Ketosis Alters Transcriptional Adaptations of Subcutaneous White Adipose Tissue in Holstein Cows during the Transition Period.Animals (Basel). 2022 Aug 30;12(17):2238. doi: 10.3390/ani12172238. Animals (Basel). 2022. PMID: 36077956 Free PMC article.
-
The Complex Interplay of Insulin Resistance and Metabolic Inflammation in Transition Dairy Cows.Animals (Basel). 2024 Mar 8;14(6):832. doi: 10.3390/ani14060832. Animals (Basel). 2024. PMID: 38539930 Free PMC article. Review.
References
-
- Herdt TH. Ruminant adaptation to negative energy balance. Influences on the etiology of ketosis and fatty liver. Veterinary Clinics of North America: Food Animal Practice. 2000;16:215–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

