SIR-Spheres yttrium-90 radioembolization for the treatment of unresectable liver cancers
- PMID: 30190962
- PMCID: PMC6095426
- DOI: 10.2217/hep.14.6
SIR-Spheres yttrium-90 radioembolization for the treatment of unresectable liver cancers
Abstract
Transarterial radioembolization with yttrium-90 resin microspheres (SIR-Spheres; Sirtex Medical Limited, Sydney, Australia) is a liver-directed therapy that is gaining recognition as a treatment option for liver-dominant primary and metastatic cancers. The incidence of complications is low and can be further reduced by patient selection and rigorous pretreatment assessment. Ideal candidates for radioembolization have preserved liver function without ascites or encephalopathy, Child-Pugh score <7 and limited lung shunting. Phase III randomized controlled trials (RCTs) against other liver-directed therapies are lacking for intermediate-stage hepatocellular carcinoma. However, preliminary data from a recent RCT has suggested that radioembolization has a similar time-to-progression and comparable toxicity to selective chemoembolization. Phase II/III RCTs are now ongoing to evaluate the combination of radioembolization with systemic therapies in advanced-stage hepatocellular carcinoma and metastatic liver-dominant colorectal cancer in order to expand the treatment opportunities for patients with cancers in the liver.
Keywords: colorectal cancer; hepatocellular carcinoma; liver metastases; neuroendocrine tumors; radioembolization; yttrium-90.
Conflict of interest statement
Financial & competing interests disclosure R Golfieri has previously received speaker fees form Bayer, Boston Scientific and Sirtex. In addition to the peer-review process, with the author's consent, the manufacturer of the product discussed in this article was given the opportunity to review the manuscript for factual accuracy. Changes were made at the discretion of the author and based on scientific or editorial merit only. The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Figures


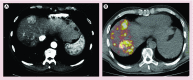
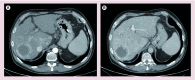
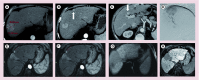


References
-
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics. CA Cancer J. Clin. 2005;55:74–108. - PubMed
-
- Bosch FX, Ribes J, Diaz M, et al. Primary liver cancer, worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16. - PubMed
-
- Lewandowski RJ, Thurston KG, Goin JE, et al. 90Y microsphere (TheraSphere) treatment for unresectable colorectal cancer metastases of the liver: response to treatment at targeted doses of 135–150 Gy as measured by [18F]fluorodeoxyglucose positron emission tomography and computed tomographic imaging. J. Vasc. Interv. Radiol. 2005;16:1641–1651. - PubMed
-
- Yao FY, Bass NM, Nikolai B, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma, implications for the current organ allocation policy. Liver Transpl. 2003;9:684–692. - PubMed
-
- Maddala YK, Stadheim L, Andrews JC, et al. Drop-out rates of patients with hepatocellular cancer listed for liver transplantation, outcome with chemoembolization. Liver Transpl. 2004;10:449–455. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
