A systematic review and meta-analysis of metal versus plastic stents for drainage of pancreatic fluid collections: metal stents are advantageous
- PMID: 30191310
- PMCID: PMC6484810
- DOI: 10.1007/s00464-018-6416-5
A systematic review and meta-analysis of metal versus plastic stents for drainage of pancreatic fluid collections: metal stents are advantageous
Abstract
Background: The use of fully covered metal stents (FCSEMS) and specifically designed lumen apposing metal stents for transmural drainage of pancreatic fluid collections has become widespread. A systematic review published in 2015 did not support the routine use of metal stents for drainage of pancreatic fluid collections. However, recent studies have shown conflicting data; therefore a systematic review and meta-analysis was performed.
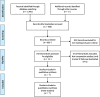
Method: We conducted a database search for original comparative studies between plastic and metal stents. The random effects model was used to calculate pooled risk ratios (RR) with 95% confidence intervals (CI). Outcomes analysed were clinical success, adverse events and requirement of further intervention.
Results: The search identified 936 studies, 7 studies with 681 (340 metal, 341 plastic) patients met inclusion criteria and were included in the meta-analysis. Clinical success was achieved in 93.8% versus 86.2% in the metal and plastic groups, respectively, RR 1.08 [95% CI 1.02-1.14]; p = 0.009. Adverse events were reduced for metal stents when compared with plastic (10.2% vs. 25.0%), RR 0.42 [95% CI 0.22-0.81]; p = 0.010. Metal stent usage reduced bleeding (2.8% vs. 7.9%), RR 0.37; [95% CI 0.18-0.75]; p = 0.006. Further intervention was required in 12.4% of patients in the metal stent group versus 26.7% for plastic stents, RR 0.54; [95% CI 0.22-1.29]; p = 0.165.
Conclusions: The use of metal stents for drainage of pancreatic fluid collections is associated with improved clinical success, fewer adverse events and reduced bleeding compared to plastic stents.
Keywords: Drainage; Endoscopic ultrasound intervention; Metal stents; Pancreatic fluid collection; Pancreatic pseudocyst; Plastic stents.
Conflict of interest statement
Prof. Neoptolemos discloses personal fees from AMGEN and Mylan; grants from Taiho Pharma (Japan), KAEL GemVax (Korea), AstraZeneca, Clovis Oncology and Ventana, Pharma Nord and NUCANA; personal fees from Boehringer Ingelheim Pharma GmbH & Co. KG, Novartis Pharma AG, KAEL GemVax, and Astellas. Prof. Sutton discloses grants from Cypralis Research, GlaxoSmithKline, Innovate UK (with Cypralis Ltd) and Merck/MSD: Supply of drug for trial (other aspects of trial funded by UK MRC and NIHR). Dr Ramesh is a Consultant for Boston Scientific. Miss Saunders, Miss Cicconi, Dr Evans, Mr Yip, Mr Raraty, Prof Ghaneh and Mr Halloran have no conflicts of interest or financial ties to disclose.
Figures







References
-
- Guidelines WGIAAP IAP/APA evidence-based guidelines for the management of acute pancreatitis. Pancreatology. 2013;13(4 Suppl 2):e1–e15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

