Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: a prospective cohort study
- PMID: 30191335
- PMCID: PMC6445809
- DOI: 10.1007/s00787-018-1219-8
Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: a prospective cohort study
Abstract
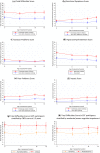
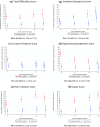
To investigate trajectories of behavior, attention, social and emotional problems to early adulthood in extremely preterm survivors compared to a term-born comparison group. Longitudinal analysis of a prospective, population-based cohort of 315 surviving infants born < 26 completed weeks of gestation recruited at birth in 1995, from the UK/Republic of Ireland, and a term-born comparison group recruited at age 6. The parent-report Strengths and Difficulties Questionnaire was completed at age 6, 11, 16 and 19 years. The Total Behavioral Difficulties Score was 4.81 points higher in extremely preterm individuals compared to their term-born peers over the period (95% CI 3.76-5.87, p < 0.001) and trajectories were stable in both groups. The impact of difficulties on home life, friendships, school or work and/or leisure activities was greater in the EPT group (RR 4.28, 95% CI 2.89-6.35, p < 0.001), and hyperactivity/inattention and peer problems accounted for the largest differences. A clinically significant behavioral screen at age 2.5 was associated with a higher Total Behavioral Difficulties Score from 6 years onwards in extremely preterm participants (Mean difference 6.90, 95% CI 5.01-8.70, p < 0.0.01), as was moderate/severe cognitive impairment at last assessment (Mean difference: 4.27, 95% CI 2.76-5.77, p < 0.001). Attention, social and emotional problems in extremely preterm individuals persist into early adulthood with significant impact on daily life. A positive behavioral screen in infancy and moderate/severe cognitive impairment are associated with early adult outcomes.
Keywords: Adolescent behavior; Child behavior; Cohort analysis; Extremely premature; Follow-up studies.
Conflict of interest statement
Conflict of interest
The sponsor, funder and authors have no conflicts of interest to disclose.
Ethical approval
No ethical approval was required for this study as it was based on data already collected for the EPICure Study.
Informed consent
Full informed parental consent was obtained for all participants recruited to the EPICure Study.
Figures
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

