Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia
- PMID: 30191360
- PMCID: PMC6510884
- DOI: 10.1007/s10456-018-9646-1
Pazopanib may reduce bleeding in hereditary hemorrhagic telangiectasia
Abstract
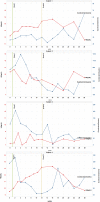
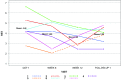
Pazopanib (Votrient) is an orally administered tyrosine kinase inhibitor that blocks VEGF receptors potentially serving as anti-angiogenic treatment for hereditary hemorrhagic telangiectasia (HHT). We report a prospective, multi-center, open-label, dose-escalating study [50 mg, 100 mg, 200 mg, and 400 mg], designed as a proof-of-concept study to demonstrate efficacy of pazopanib on HHT-related bleeding, and to measure safety. Patients, recruited at 5 HHT Centers, required ≥ 2 Curacao criteria AND [anemia OR severe epistaxis with iron deficiency]. Co-primary outcomes, hemoglobin (Hgb) and epistaxis severity, were measured during and after treatment, and compared to baseline. Safety monitoring occurred every 1.5 weeks. Seven patients were treated with 50 mg pazopanib daily. Six/seven showed at least 50% decrease in epistaxis duration relative to baseline at some point during study; 3 showed at least 50% decrease in duration during Weeks 11 and 12. Six patients showed a decrease in ESS of > 0.71 (MID) relative to baseline at some point during study; 3/6 showed a sustained improvement. Four patients showed > 2 gm improvement in Hgb relative to baseline at one or more points during study. Health-related QOL scores improved on all SF-36 domains at Week 6 and/or Week 12, except general health (unchanged). There were 19 adverse events (AE) including one severe AE (elevated LFTs, withdrawn from dosing at 43 days); with no serious AE. In conclusion, we observed an improvement in Hgb and/or epistaxis in all treated patients. This occurred at a dose much lower than typically used for oncologic indications, with no serious AE. Further studies of pazopanib efficacy are warranted.
Keywords: Anemia; Epistaxis; Hereditary; Telangiectasia; Tyrosine kinase inhibitor.
Conflict of interest statement
MF reports grants from GSK, during the conduct of the study; personal fees from HHT Foundation International (Cure HHT), grants from NIH-NINDS-NCATS, grants from NIH-NHLBI, grants from US Department of Defense, outside the submitted work; MCM reports grants from GSK, during the conduct of the study; grants from HHT Foundation International, personal fees from Express Scripts International, outside the submitted work. JD is a GSK employee and share holder. GP reports other from Novartis, during the conduct of the study; other from GSK, outside the submitted work; PB reports other from GSK, other from Janssen Global Services, outside the submitted work. DLS reports other from GlaxoSmithKline, outside the submitted work; and I have worked with the HHT patient advocacy group for many years, but receive no monies related to that activity. However, this has motivated my interest in pursuing this work; JG, SPO, RK, CCWH, JM, JGP, NV have nothing to disclose.
Figures

References
-
- Donaldson JW, McKeever TM, Hall IP, Hubbard RB, Fogarty AW. The UK prevalence of hereditary haemorrhagic telangiectasia and its association with sex, socioeconomic status and region of residence: a population-based study. Thorax. 2014;69(2):161–167. doi: 10.1136/thoraxjnl-2013-203720. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

