Treatment Duration, Healthcare Resource Utilization, and Costs Among Chemotherapy-Naïve Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide or Abiraterone Acetate: A Retrospective Claims Analysis
- PMID: 30191463
- PMCID: PMC6182626
- DOI: 10.1007/s12325-018-0774-1
Treatment Duration, Healthcare Resource Utilization, and Costs Among Chemotherapy-Naïve Patients with Metastatic Castration-Resistant Prostate Cancer Treated with Enzalutamide or Abiraterone Acetate: A Retrospective Claims Analysis
Abstract
Introduction: Enzalutamide and abiraterone acetate (plus prednisone) are new hormonal treatments for metastatic castration-resistant prostate cancer (mCRPC). This study compared treatment duration, healthcare resource utilization (HRU), and treatment costs for chemotherapy-naïve mCRPC patients treated with enzalutamide or abiraterone acetate in the USA.
Methods: Chemotherapy-naïve mCRPC patients initiating treatment with enzalutamide or abiraterone acetate were identified from administrative claims. Continuous enrollment ≥ 6 months before and ≥ 3 months after the index date (initiation date of enzalutamide or abiraterone acetate) was required. Treatment duration, all-cause and prostate cancer-related HRU, and costs were estimated during the post-index period. Multivariable analyses compared HRU and costs between cohorts, adjusting for baseline characteristics.
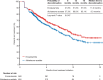
Results: Overall, 920 chemotherapy-naïve patients initiated enzalutamide and 2310 initiated abiraterone acetate (median follow-up, 10.7 and 13.5 months, respectively). More enzalutamide-treated patients had corticosteroid-sensitive comorbidities at baseline. Treatment duration was longer with enzalutamide versus abiraterone acetate (median, 10.7 vs. 8.8 months; P = 0.008). Enzalutamide was associated with fewer all-cause inpatient admissions [adjusted incidence rate ratio (95% confidence interval) 0.87 (0.76, 0.99)], days of hospitalization [0.84 (0.70, 1.02)], and outpatient visits [0.94 (0.90, 0.98)], and fewer prostate cancer-related outpatient visits [0.92 (0.87, 0.96)] compared with abiraterone acetate. Enzalutamide was also associated with lower prostate cancer-related inpatient and emergency department costs [adjusted differences, $122 (P = 0.024) and $28 (P = 0.009), respectively].
Conclusion: Chemotherapy-naïve mCRPC patients treated with enzalutamide versus abiraterone acetate had longer treatment duration and incurred lower HRU and prostate cancer-related inpatient and emergency department costs.
Funding: Astellas Pharma Inc.
Keywords: Abiraterone acetate; Chemotherapy naïve; Claims analysis; Enzalutamide; Healthcare costs; Hormonal therapy; Metastatic castration-resistant prostate cancer; Oncology; Retrospective study; Treatment patterns.
Conflict of interest statement
Neil M. Schultz is an employee of Astellas Pharma Inc. and owns stock in Gilead Sciences and Shire. Samuel Wilson is an employee of Astellas Pharma Inc. Scott C. Flanders was an employee of Astellas Pharma Inc. when the research was conducted and is now affiliated with Dendreon Pharmaceuticals LLC and owns stock in Johnson & Johnson and AbbVie. Bruce A. Brown was an employee of Astellas Pharma Inc. when the research was conducted and is now affiliated with Dendreon Pharmaceuticals LLC. Yan Song is an employee of Analysis Group, Inc., which has received consultancy fees from Astellas Pharma Inc. Hongbo Yang is an employee of Analysis Group, Inc., which has received consultancy fees from Astellas Pharma Inc. Stanislav Lechpammer is an employee of Pfizer Inc. Vahan Kassabian is a speaker for Astellas/Pfizer, Janssen, Dendreon, Amgen, and Bayer; a consultant for Dendreon, UroGPO, Bayer, Tolmar, Janssen, Astellas/Pfizer; an employee of Georgia Urology; and owns UroGPO stock.
Figures


References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase no. 11. 2014. http://globocan.iarc.fr. Accessed 22 Mar 2018.
-
- National Cancer Institute. Prostate cancer treatment-health professional version (PDQ®). 2018. http://www.cancer.gov/types/prostate/hp/prostate-treatment-pdq. Accessed 7 Jul 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources

