A new era for stroke therapy: Integrating neurovascular protection with optimal reperfusion
- PMID: 30191760
- PMCID: PMC6282224
- DOI: 10.1177/0271678X18798162
A new era for stroke therapy: Integrating neurovascular protection with optimal reperfusion
Abstract
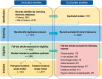
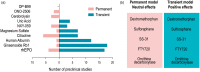
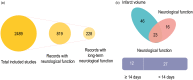
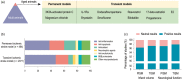
Recent advances in stroke reperfusion therapies have led to remarkable improvement in clinical outcomes, but many patients remain severely disabled, due in part to the lack of effective neuroprotective strategies. In this review, we show that 95% of published preclinical studies on "neuroprotectants" (1990-2018) reported positive outcomes in animal models of ischemic stroke, while none translated to successful Phase III trials. There are many complex reasons for this failure in translational research, including that the majority of clinical trials did not test early delivery of neuroprotectants in combination with successful reperfusion. In contrast to the clinical trials, >80% of recent preclinical studies examined the neuroprotectant in animal models of transient ischemia with complete reperfusion. Furthermore, only a small fraction of preclinical studies included long-term functional assessments, aged animals of both genders, and models with stroke comorbidities. Recent clinical trials demonstrate that 70%-80% of patients treated with endovascular thrombectomy achieve successful reperfusion. These successes revive the opportunity to retest previously failed approaches, including cocktail drugs that target multiple injury phases and different cell types. It is our hope that neurovascular protectants can be retested in future stroke research studies with specific criteria outlined in this review to increase translational successes.
Keywords: Stroke; ischemia; neuroprotection; reperfusion; translational research.
Figures
References
-
- National Institute of Neurological Disorders, Stroke rt PA Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 1995; 333: 1581–1587. - PubMed
-
- Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–1329. - PubMed
-
- Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The proact ii study: A randomized controlled trial. Prolyse in acute cerebral thromboembolism. Jama 1999; 282: 2003–2011. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

