Dual-Function RNAs
- PMID: 30191807
- PMCID: PMC6130917
- DOI: 10.1128/microbiolspec.RWR-0032-2018
Dual-Function RNAs
Abstract
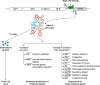
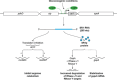
Bacteria are known to use RNA, either as mRNAs encoding proteins or as noncoding small RNAs (sRNAs), to regulate numerous biological processes. However, a few sRNAs have two functions: they act as base-pairing RNAs and encode a small protein with additional regulatory functions. Thus, these so called "dual-function" sRNAs can serve as both a riboregulator and an mRNA. In some cases, these two functions can act independently within the same pathway, while in other cases, the base-pairing function and protein function act in different pathways. Here, we discuss the five known dual-function sRNAs-SgrS from enteric species, RNAIII and Psm-mec from Staphylococcus aureus, Pel RNA from Streptococcus pyogenes, and SR1 from Bacillus subtilis-and review their mechanisms of action and roles in regulating diverse biological processes. We also discuss the prospect of finding additional dual-function sRNAs and future challenges in studying the overlap and competition between the functions.
Figures



Similar articles
-
Dual-function RNA regulators in bacteria.Biochimie. 2011 Nov;93(11):1943-9. doi: 10.1016/j.biochi.2011.07.016. Epub 2011 Jul 24. Biochimie. 2011. PMID: 21816203 Free PMC article. Review.
-
Dual-function small regulatory RNAs in bacteria.Mol Microbiol. 2017 Feb;103(3):387-397. doi: 10.1111/mmi.13558. Epub 2016 Nov 14. Mol Microbiol. 2017. PMID: 27750368 Review.
-
Base pairing small RNAs and their roles in global regulatory networks.FEMS Microbiol Rev. 2010 Sep;34(5):866-82. doi: 10.1111/j.1574-6976.2010.00241.x. Epub 2010 Jun 23. FEMS Microbiol Rev. 2010. PMID: 20662934 Free PMC article. Review.
-
Mechanistic study of base-pairing small regulatory RNAs in bacteria.Methods. 2017 Mar 15;117:67-76. doi: 10.1016/j.ymeth.2016.09.012. Epub 2016 Sep 28. Methods. 2017. PMID: 27693881 Review.
-
Dynamic interactions between the RNA chaperone Hfq, small regulatory RNAs, and mRNAs in live bacterial cells.Elife. 2021 Feb 22;10:e64207. doi: 10.7554/eLife.64207. Elife. 2021. PMID: 33616037 Free PMC article.
Cited by
-
cncRNAdb: a manually curated resource of experimentally supported RNAs with both protein-coding and noncoding function.Nucleic Acids Res. 2021 Jan 8;49(D1):D65-D70. doi: 10.1093/nar/gkaa791. Nucleic Acids Res. 2021. PMID: 33010163 Free PMC article.
-
Small regulatory RNAs in Vibrio cholerae.Microlife. 2023 Jun 15;4:uqad030. doi: 10.1093/femsml/uqad030. eCollection 2023. Microlife. 2023. PMID: 37441523 Free PMC article. Review.
-
LipR functions as an intracellular pH regulator in Bacillus thuringiensis under glucose conditions.mLife. 2023 Feb 11;2(1):58-72. doi: 10.1002/mlf2.12055. eCollection 2023 Mar. mLife. 2023. PMID: 38818337 Free PMC article.
-
Investigation of the global translational response to oxidative stress in the model archaeon Haloferax volcanii reveals untranslated small RNAs with ribosome occupancy.bioRxiv [Preprint]. 2025 Jul 13:2025.04.08.647799. doi: 10.1101/2025.04.08.647799. bioRxiv. 2025. PMID: 40672279 Free PMC article. Preprint.
-
There's Plenty of Room Right Here: Biological Systems as Evolved, Overloaded, Multi-Scale Machines.Biomimetics (Basel). 2023 Mar 8;8(1):110. doi: 10.3390/biomimetics8010110. Biomimetics (Basel). 2023. PMID: 36975340 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

