Elucidating the kinetics of sodium fluorescein for fluorescence-guided surgery of glioma
- PMID: 30192200
- PMCID: PMC6995036
- DOI: 10.3171/2018.4.JNS172644
Elucidating the kinetics of sodium fluorescein for fluorescence-guided surgery of glioma
Abstract
Objective: The use of the optical contrast agent sodium fluorescein (NaFl) to guide resection of gliomas has been under investigation for decades. Although this imaging strategy assumes the agent remains confined to the vasculature except in regions of blood-brain barrier (BBB) disruption, clinical studies have reported significant NaFl signal in normal brain tissue, limiting tumor-to-normal contrast. A possible explanation arises from earlier studies, which reported that NaFl exists in both pure and protein-bound forms in the blood, the former being small enough to cross the BBB. This study aims to elucidate the kinetic binding behavior of NaFl in circulating blood and its effect on NaFl accumulation in brain tissue and tumor contrast. Additionally, the authors examined the blood and tissue kinetics, as well as tumor uptake, of a pegylated form of fluorescein selected as a potential optical analog of gadolinium-based MRI contrast agents.
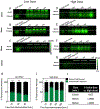
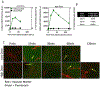
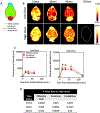
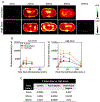
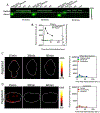
Methods: Cohorts of mice were administered one of the following doses/forms of NaFl: 1) high human equivalent dose (HED) of NaFl, 2) low HED of NaFl, or 3) pegylated form of fluorescein. In each cohort, groups of animals were euthanized 15, 30, 60, and 120 minutes after administration for ex vivo analysis of fluorescein fluorescence. Using gel electrophoresis and fluorescence imaging of blood and brain specimens, the authors quantified the temporal kinetics of bound NaFl, unbound NaFl, and pegylated fluorescein in the blood and normal brain tissue. Finally, they compared tumor-to-normal contrast for NaFl and pegylated-fluorescein in U251 glioma xenografts.
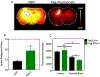
Results: Administration of NaFl resulted in the presence of unbound and protein-bound NaFl in the circulation, with unbound NaFl constituting up to 70% of the signal. While protein-bound NaFl was undetectable in brain tissue, unbound NaFl was observed throughout the brain. The observed behavior was time and dose dependent. The pegylated form of fluorescein showed minimal uptake in brain tissue and improved tumor-to-normal contrast by 38%.
Conclusions: Unbound NaFl in the blood crosses the BBB, limiting the achievable tumor-to-normal contrast and undermining the inherent advantage of tumor imaging in the brain. Dosing and incubation time should be considered carefully for NaFl-based fluorescence-guided surgery (FGS) of glioma. A pegylated form of fluorescein showed more favorable normal tissue kinetics that translated to higher tumor-to-normal contrast. These results warrant further development of pegylated-fluorescein for FGS of glioma.
Keywords: BBB = blood-brain barrier; FGS = fluorescence-guided surgery; HED = human equivalent dose; NaFl = sodium fluorescein; PBS = phosphate-buffered saline; ROI = region of interest; blood-brain barrier; brain tumor; fluorescence-guided surgery; oncology; sodium fluorescein; surgical technique.
Conflict of interest statement
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Figures

References
-
- Acerbi F, Broggi M, Eoli M, Anghileri E, Cuppini L, Pollo B, et al.: Fluorescein-guided surgery for grade IV gliomas with a dedicated filter on the surgical microscope: preliminary results in 12 cases. Acta Neurochir (Wien) 155:1277–1286, 2013 - PubMed
-
- Delori FC, Castany MA, Webb RH: Fluorescence characteristics of sodium fluorescein in plasma and whole blood. Exp Eye Res 27:417–425, 1978 - PubMed
-
- Dimou S, Battisti RA, Hermens DF, Lagopoulos J: A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg Rev 36:205–214, 2013 - PubMed
-
- Ding R, Frei E, Fardanesh M, Schrenk HH, Kremer P, Haefeli WE: Pharmacokinetics of 5-aminofluorescein-albumin, a novel fluorescence marker of brain tumors during surgery. J Clin Pharmacol 51:672–678, 2011 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

