A Phase 1, Multiple-Dose Study of Vedolizumab in Japanese Patients With Ulcerative Colitis
- PMID: 30192378
- PMCID: PMC6718004
- DOI: 10.1002/jcph.1307
A Phase 1, Multiple-Dose Study of Vedolizumab in Japanese Patients With Ulcerative Colitis
Abstract
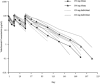
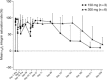
Although previous studies have shown that patients with ulcerative colitis may benefit from treatment with vedolizumab, a humanized monoclonal antibody targeting the α4 β7 integrin heterodimer, no data exist in Japanese populations. The aim of this phase 1, open-label, multicenter study was to assess the pharmacokinetics, pharmacodynamics, efficacy, and safety of vedolizumab in Japanese patients with ulcerative colitis. Adult patients with confirmed ulcerative colitis received either 150 mg (step 1) or 300 mg (step 2) of intravenous (IV) vedolizumab on days 1, 15, and 43 of the study protocol. Pharmacokinetic, pharmacodynamic, safety, and efficacy parameters were all assessed through study end (day 239). Nine patients were enrolled in this study (150 mg, n = 3; 300 mg, n = 6). Patients who received vedolizumab IV 300 mg had approximately twice the drug exposure of those receiving vedolizumab IV 150 mg (day 1 AUCday14 744 vs 408 μg·d/mL) and a longer-lasting maximal saturation of α4 β7 integrin (155 vs 99 days). The number of treatment-emergent adverse events, all of which were mild or moderate in intensity, was similar between the 150-mg (15 events) and 300-mg (20 events) groups. The 2 patients (150 mg group) not in clinical remission by partial Mayo score at the start of the study met the criteria for clinical remission on days 15 and 155 of the study, respectively. In conclusion, in Japanese patients with ulcerative colitis, vedolizumab showed similar pharmacokinetic and pharmacodynamic results to those seen in non-Japanese patients. Vedolizumab was well tolerated and demonstrated clinical activity consistent with previous studies.
Keywords: Japanese; inflammatory bowel disease; ulcerative colitis; vedolizumab.
© 2018, The American College of Clinical Pharmacology.
Figures
References
-
- Matsuoka K, Lee T‐C. Guidelines for the management of ulcerative colitis in Japan—developed through integration of evidence and consensus among experts. IBD Res. 2010;4:189–239.
-
- Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380(9853):1606–1619. - PubMed
-
- Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(7):390–407. - PubMed
-
- Asakura K, Nishiwaki Y, Inoue N, Hibi T, Watanabe M, Takebayashi T. Prevalence of ulcerative colitis and Crohn's disease in Japan. J Gastroenterol. 2009;44(7):659–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

