Angiomyolipoma rebound tumor growth after discontinuation of everolimus in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis
- PMID: 30192751
- PMCID: PMC6128468
- DOI: 10.1371/journal.pone.0201005
Angiomyolipoma rebound tumor growth after discontinuation of everolimus in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis
Abstract
Introduction: The EXIST-2 (NCT00790400) study demonstrated the superiority of everolimus over placebo for the treatment of renal angiomyolipomas associated with tuberous sclerosis complex (TSC) or sporadic lymphangioleiomyomatosis (LAM). This post hoc analysis of EXIST-2 study aimed to assess angiomyolipoma tumor behavior among patients who submitted to continued radiographic examination following discontinuation of everolimus in the noninterventional follow-up phase.
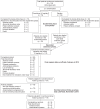
Methods: For patients who discontinued everolimus at the completion of extension phase for reasons other than angiomyolipoma progression, a single CT/MRI scan of the kidney was collected after 1 year of treatment discontinuation. Changes from baseline and from the time of everolimus discontinuation in the sum of volumes of target angiomyolipoma lesions were assessed in the non-interventional follow-up phase (data cutoff date, November 6, 2015).
Results: Of the 112 patients who received ≥1 dose of everolimus and discontinued treatment by the end of extension phase, 34 (30.4%) were eligible for participation in the non-interventional follow-up phase. Sixteen of 34 patients were evaluable for angiomyolipoma tumor behavior as they had at least one valid efficacy assessment (i.e. kidney CT/MRI scan) after everolimus discontinuation. During the non-interventional follow-up phase, compared with baseline, two patients (12.5%) experienced angiomyolipoma progression (angiomyolipoma-related bleeding [n = 1], increased kidney volume [n = 1]). Five patients out of 16 (31.3%) experienced angiomyolipoma progression when compared with the angiomyolipoma tumor assessment at everolimus discontinuation. The median (range) percentage change in angiomyolipoma tumor volume (cm3) from baseline was -70.56 (-88.30; -49.64) at time of everolimus discontinuation (n = 11), and -50.55 (-79.40; -23.16) at week 48 (n = 7) after discontinuation of everolimus. One patient death was reported due to angiomyolipoma hemorrhage.
Conclusions: Angiomyolipoma lesions displayed an increase in volume following discontinuation of everolimus in patients with renal angiomyolipoma or sporadic LAM associated with TSC, but there was no evidence of rapid regrowth.
Trial registration: ClinicalTrials.gov NCT00790400.
Conflict of interest statement
JJB, NN, EB, MB, MF, KB have served as investigators on this study and received research grants (to their institutions) from Novartis. JJB, EB, KB have served as consultants and/or participated in advisory boards for Novartis. JJB, NN, PC, KB, MF have received travel honoraria from Novartis. BAZ has received research grants, was part of advisory boards, and received travel grants from Novartis. AZ has nothing to disclose. MV, AL, and FH are employees of Novartis. FH owns stock in Novartis. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus G. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243–54. 10.1016/j.pediatrneurol.2013.08.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

