Impaired hepatic amyloid-beta degradation in Alzheimer's disease
- PMID: 30192871
- PMCID: PMC6128628
- DOI: 10.1371/journal.pone.0203659
Impaired hepatic amyloid-beta degradation in Alzheimer's disease
Abstract
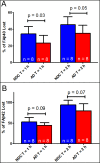
Extensive research strongly suggests that amyloid beta (Aβ) aggregates in the brain have a central role in Alzheimer's disease (AD) pathogenesis. Pathological Aβ deposition is likely due to an altered balance between overproduction and elimination. Rodent studies have suggested that the liver has a major role in Aβ degradation. It is possible alterations of liver function could affect brain Aβ levels through changes in blood Aβ concentration. In this study, we hypothesized hepatic Aβ degradation to be impaired in AD subjects. To test our hypothesis, an Aβ degradation assay was developed using synthetic fluorescein-labeled Aβ40 and Aβ42 spiked into human liver homogenates. Aβ degradation rates were lower in AD-derived homogenates as compared with those from non-demented (ND) control subjects, even after accounting for such covariates as age, sex, and APOE genotype. The protein expression of potential Aβ-degrading enzymes were also examined. Neprilysin levels were not different in AD liver samples, while cathepsin D and insulin-degrading enzyme were significantly altered in AD subjects. The results support the possibility that impaired hepatic Aβ degradation could be a factor contributing to increased brain Aβ accumulation and AD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Tanzi RE. Molecular genetics of Alzheimer's disease and the amyloid beta peptide precursor gene. Ann Med 1989;21(2):91–4. - PubMed
-
- Hersh LB, Rodgers DW. Neprilysin and amyloid beta peptide degradation. Curr Alzheimer Res 2008. April;5(2):225–31. - PubMed
-
- Howell S, Nalbantoglu J, Crine P. Neutral endopeptidase can hydrolyze beta-amyloid(1–40) but shows no effect on beta-amyloid precursor protein metabolism. Peptides 1995;16(4):647–52. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

