Effect of Mobile Device-Supported Single-Patient Multi-crossover Trials on Treatment of Chronic Musculoskeletal Pain: A Randomized Clinical Trial
- PMID: 30193253
- PMCID: PMC6233756
- DOI: 10.1001/jamainternmed.2018.3981
Effect of Mobile Device-Supported Single-Patient Multi-crossover Trials on Treatment of Chronic Musculoskeletal Pain: A Randomized Clinical Trial
Abstract
Importance: Individually designed single-patient multi-crossover (n-of-1) trials can facilitate tailoring of treatments directed at various conditions, including chronic musculoskeletal pain (CMSP) but are potentially burdensome, which may limit uptake in research and practice.
Objectives: To determine whether patients randomized to participate in an n-of-1 trial supported by a mobile health (mHealth) app would experience less pain and improved global health, adherence, satisfaction, and shared decision making compared with patients assigned to usual care.
Design, setting, and participants: This randomized clinical trial compared participation in an individualized, mHealth-supported n-of-1 trial vs usual care. The participating 215 patients had CMSP for at least 6 weeks, had a smartphone or tablet with a data plan, were enrolled in northern California from July 2014 through July 2016, and were followed for up to 1 year by 48 clinicians in academic, community, Veterans Affairs, and military settings.
Interventions: Intervention patients met with their clinicians and used a desktop interface to select treatments and trial parameters for an n-of-1 trial comparing 2 pain-management regimens. The mHealth app provided reminders to take designated treatments on assigned days and to upload responses to daily questions on pain and treatment-associated adverse effects. Control patients received care as usual.
Main outcomes and measures: The primary outcome was change in the PROMIS (Patient-Reported Outcomes Measurement Information System) pain-related interference 8-item short-form scale (full scale range, 41-78) from baseline to 6 months. Secondary outcomes included patient-reported pain intensity, overall health, analgesic adherence, trust in clinician, satisfaction with care, medication-related shared decision making, and, for the n-of-1 group only, participant engagement and experience.
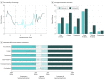
Results: Among 215 patients (108 randomized to the n-of-1 intervention and 107 to control), 102 (47%) were women, and the mean (SD) age was 55.5 (11.1) years. At the 6-month follow-up, pain interference was reduced in both groups, though there was no difference between the intervention and control groups (-1.36 points; 95% CI, -2.91 to 0.19 points; P = .09). There were no advantages in secondary outcomes for intervention patients vs control patients except for higher medication-related shared decision making at 6 months (between-group difference, 11.9 points; 95% CI, 2.6-21.2 points; P = .01). Among patients assigned to the n-of-1 group, 88% (n = 86) affirmed that the mHealth app could help people like them manage their pain.
Conclusions and relevance: In this population of patients with CMSP, mHealth-supported n-of-1 trials were feasible and associated with a satisfactory user experience, but n-of-1 trial participation did not significantly improve pain interference at 6 months vs usual care.
Trial registration: ClinicalTrials.gov identifier: NCT02116621.
Conflict of interest statement
Figures
Comment in
-
A Randomized Clinical Trial of n-of-1 Trials-Tribulations of a Trial.JAMA Intern Med. 2018 Oct 1;178(10):1378-1379. doi: 10.1001/jamainternmed.2018.3979. JAMA Intern Med. 2018. PMID: 30193302 No abstract available.
-
A Case for n-of-1 Trials.JAMA Intern Med. 2019 Mar 1;179(3):452. doi: 10.1001/jamainternmed.2018.7166. JAMA Intern Med. 2019. PMID: 30830183 No abstract available.
-
A Case for n-of-1 Trials.JAMA Intern Med. 2019 Mar 1;179(3):452-453. doi: 10.1001/jamainternmed.2018.7183. JAMA Intern Med. 2019. PMID: 30830184 No abstract available.
-
Finding Benefit in n-of-1 Trials.JAMA Intern Med. 2019 Mar 1;179(3):453-454. doi: 10.1001/jamainternmed.2018.8379. JAMA Intern Med. 2019. PMID: 30830189 No abstract available.
-
Finding Benefit in n-of-1 Trials.JAMA Intern Med. 2019 Mar 1;179(3):454-455. doi: 10.1001/jamainternmed.2018.8382. JAMA Intern Med. 2019. PMID: 30830190 No abstract available.
-
A Case for n-of-1 Trials-Reply.JAMA Intern Med. 2019 Mar 1;179(3):453. doi: 10.1001/jamainternmed.2018.7180. JAMA Intern Med. 2019. PMID: 30830192 No abstract available.
-
Finding Benefit in n-of-1 Trials-Reply.JAMA Intern Med. 2019 Mar 1;179(3):455. doi: 10.1001/jamainternmed.2018.8330. JAMA Intern Med. 2019. PMID: 30830195 No abstract available.
References
-
- Cook DJ. Randomized trials in single subjects: the N of 1 study. Psychopharmacol Bull. 1996;32(3):363-367. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

