The efficacy and safety of combined administration of intravenous and topical tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials
- PMID: 30193586
- PMCID: PMC6129000
- DOI: 10.1186/s12891-018-2181-9
The efficacy and safety of combined administration of intravenous and topical tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled trials
Abstract
Background: The combined administration of intravenous (IV) and topical tranexamic acid (TXA) in primary total knee (TKA) knee remains controversial. The purpose of this meta-analysis was to assess the efficacy and safety of combined administration of IV and topical TXA in primary TKA.
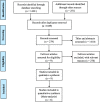
Methods: PubMed, EMBASE, Cochrane Central Register of Controlled Trials, Web of Science, Google Search Engine and China National Knowledge Infrastructure databases were searched for randomized controlled trials (RCTs) were comparing the combined administration of IV and topical TXA following primary TKA. The primary outcomes were total blood loss, maximum hemoglobin drop, and deep venous thrombosis (DVT) and/or pulmonary embolism (PE). The second outcomes were drainage volume and transfusion requirements. Data were analyzed using RevMan 5.3.
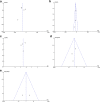
Results: A total of 6 RCTs involving 701 patients were included in the meta-analysis. The combined group provided lower total blood loss (MD - 156.34 mL, 95% CI, - 241.51 to - 71.18; P = 0.0003), drainage volume (MD - 43.54 mL, 95% CI, - 67.59 to - 19.48; P = 0.0004), maximum hemoglobin drop (MD - 0.56 g/dl, 95% CI, - 0.93 to - 0.19; P = 0.003) than IV TXA alone. No significant difference were found in terms of transfusion requirements (RR 0.48, 95% CI, 0.16 to 1.44; P = 0.19), DVT (RR 1.01, 95% CI, 0.14 to 7.12; P = 0.99) and PE (RR 0.33, 95% CI, 0.01 to 7.91; P = 0.49) between the two group. Subgroup analyses shows that the combined group was less total blood loss in non-tourniquet (P = 0.0008), topical TXA dose > 1.5 g (P < 0.00001) and number of IV TXA ≥ 2 doses (P = 0.005) of TXA compared with the IV group alone.
Conclusions: The available evidence indicates combined group were associated with lower total blood loss, drainage volume, and maximum hemoglobin drop. A similar transfusion requirement was found in both groups. Subgroup analyses demonstrates that total blood loss was less in patients with non-tourniquet, topical TXA dose > 1.5 g and number of IV TXA ≥ 2 doses of TXA. There was no increase the rates of DVT and PE.
Keywords: Blood loss; Intravenous; Topical; Total knee arthroplasty; Tranexamic acid.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval is not necessary because it is a comment on previously published articles and does not involve the handling of any personal patient data.
Consent for publication
All authors agree to publish.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
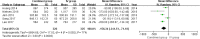
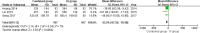
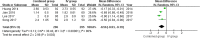
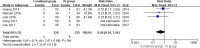

References
-
- Edelstein AI, Kwasny MJ, Suleiman LI, Khakhkhar RH, Moore MA, Beal MD, et al. Can the American College of Surgeons risk calculator predict 30-day complications after knee and hip arthroplasty? J Arthroplast 2015;30:5–10. - PubMed
-
- Wong J, Abrishami A, El Beheiry H, Mahomed NN, Roderick Davey J, Gandhi R, et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial.J Bone Joint Surg Am 2010 ;92:2503–2513. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

