Priming of Plant Growth Promotion by Volatiles of Root-Associated Microbacterium spp
- PMID: 30194105
- PMCID: PMC6210106
- DOI: 10.1128/AEM.01865-18
Priming of Plant Growth Promotion by Volatiles of Root-Associated Microbacterium spp
Abstract
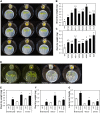
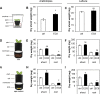
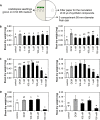
Volatile compounds produced by plant-associated microorganisms represent a diverse resource to promote plant growth and health. Here, we investigated the effect of volatiles from root-associated Microbacterium species on plant growth and development. Volatiles of eight strains induced significant increases in shoot and root biomass of Arabidopsis but differed in their effects on root architecture. Microbacterium strain EC8 also enhanced root and shoot biomass of lettuce and tomato. Biomass increases were also observed for plants exposed only briefly to volatiles from EC8 prior to transplantation of the seedlings to soil. These results indicate that volatiles from EC8 can prime plants for growth promotion without direct and prolonged contact. We further showed that the induction of plant growth promotion is tissue specific; that is, exposure of roots to volatiles from EC8 led to an increase in plant biomass, whereas shoot exposure resulted in no or less growth promotion. Gas chromatography-quadrupole time of flight mass spectometry (GC-QTOF-MS) analysis revealed that EC8 produces a wide array of sulfur-containing compounds, as well as ketones. Bioassays with synthetic sulfur volatile compounds revealed that the plant growth response to dimethyl trisulfide was concentration-dependent, with a significant increase in shoot weight at 1 μM and negative effects on plant biomass at concentrations higher than 1 mM. Genome-wide transcriptome analysis of volatile-exposed Arabidopsis seedlings showed upregulation of genes involved in assimilation and transport of sulfate and nitrate. Collectively, these results show that root-associated Microbacterium primes plants, via the roots, for growth promotion, most likely via modulation of sulfur and nitrogen metabolism.IMPORTANCE In the past decade, various studies have described the effects of microbial volatiles on other (micro)organisms in vitro, but their broad-spectrum activity in vivo and the mechanisms underlying volatile-mediated plant growth promotion have not been addressed in detail. Here, we revealed that volatiles from root-associated bacteria of the genus Microbacterium can enhance the growth of different plant species and can prime plants for growth promotion without direct and prolonged contact between the bacterium and the plant. Collectively, these results provide new opportunities for sustainable agriculture and horticulture by exposing roots of plants only briefly to a specific blend of microbial volatile compounds prior to transplantation of the seedlings to the greenhouse or field. This strategy has no need for large-scale introduction or root colonization and survival of the microbial inoculant.
Keywords: VOCs; biostimulant; plant-microbe interactions; volatile organic compounds.
Copyright © 2018 American Society for Microbiology.
Figures
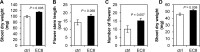

Similar articles
-
Growth promotion of Lactuca sativa in response to volatile organic compounds emitted from diverse bacterial species.Microbiol Res. 2016 Dec;193:39-47. doi: 10.1016/j.micres.2016.09.008. Epub 2016 Sep 27. Microbiol Res. 2016. PMID: 27825485
-
Soil bacterial diffusible and volatile organic compounds inhibit Phytophthora capsici and promote plant growth.Sci Total Environ. 2019 Nov 20;692:267-280. doi: 10.1016/j.scitotenv.2019.07.061. Epub 2019 Jul 5. Sci Total Environ. 2019. PMID: 31349168
-
Cladosporium psychrotolerans strain T01 enhances plant biomass and also exhibits antifungal activity against pathogens.Braz J Microbiol. 2024 Sep;55(3):2855-2867. doi: 10.1007/s42770-024-01399-7. Epub 2024 Jun 3. Braz J Microbiol. 2024. PMID: 38825649 Free PMC article.
-
Microbial volatiles as plant growth inducers.Microbiol Res. 2018 Mar;208:63-75. doi: 10.1016/j.micres.2018.01.002. Epub 2018 Jan 31. Microbiol Res. 2018. PMID: 29551213 Review.
-
Induced plant volatiles allow sensitive monitoring of plant health status in greenhouses.Plant Signal Behav. 2009 Sep;4(9):824-9. doi: 10.4161/psb.4.9.9431. Epub 2009 Sep 24. Plant Signal Behav. 2009. PMID: 19847108 Free PMC article. Review.
Cited by
-
The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture.Microorganisms. 2021 Aug 30;9(9):1841. doi: 10.3390/microorganisms9091841. Microorganisms. 2021. PMID: 34576736 Free PMC article. Review.
-
Volatile organic compounds emitted by Burkholderia pyrrocinia CNUC9 trigger induced systemic salt tolerance in Arabidopsis thaliana.Front Microbiol. 2022 Nov 17;13:1050901. doi: 10.3389/fmicb.2022.1050901. eCollection 2022. Front Microbiol. 2022. PMID: 36466674 Free PMC article.
-
Diverse roles played by "Pseudomonas fluorescens complex" volatile compounds in their interaction with phytopathogenic microrganims, pests and plants.World J Microbiol Biotechnol. 2024 Jan 28;40(3):80. doi: 10.1007/s11274-023-03873-0. World J Microbiol Biotechnol. 2024. PMID: 38281212 Free PMC article. Review.
-
Volatile organic compounds shape belowground plant-fungi interactions.Front Plant Sci. 2022 Dec 6;13:1046685. doi: 10.3389/fpls.2022.1046685. eCollection 2022. Front Plant Sci. 2022. PMID: 36561453 Free PMC article. Review.
-
Complete Genome Sequence of Microbacterium foliorum NRRL B-24224, a Host for Bacteriophage Discovery.Microbiol Resour Announc. 2019 Jan 31;8(5):e01467-18. doi: 10.1128/MRA.01467-18. eCollection 2019 Jan. Microbiol Resour Announc. 2019. PMID: 30714032 Free PMC article.
References
-
- Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC. 2005. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol Biochem 37:955–964. doi:10.1016/j.soilbio.2004.10.021. - DOI
-
- Epstein L, Lockwood JL. 1984. Effect of soil microbiota on germination of Bipolaris victoriae conidia. Trans Br Mycol Soc 82:63–69. doi:10.1016/S0007-1536(84)80212-0. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

