Endothelial cell fitness dictates the source of regenerating liver vasculature
- PMID: 30194265
- PMCID: PMC6170182
- DOI: 10.1084/jem.20180008
Endothelial cell fitness dictates the source of regenerating liver vasculature
Abstract
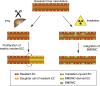
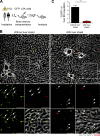
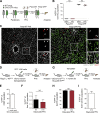
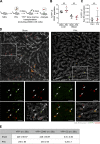
Neoangiogenesis plays a key role in diverse pathophysiological conditions, including liver regeneration. Yet, the source of new endothelial cells (ECs) remains elusive. By analyzing the regeneration of the liver vasculature in irradiation-based myeloablative and nonmyeloablative bone marrow transplantation mouse models, we discovered that neoangiogenesis in livers with intact endothelium was solely mediated by proliferation of resident ECs. However, following irradiation-induced EC damage, bone marrow-derived mononuclear cells were recruited and incorporated into the vasculature. Further experiments with direct bone marrow infusion or granulocyte colony-stimulating factor (G-CSF)-mediated progenitor cell mobilization, which resembles clinically relevant stem cell therapy, demonstrated that bone marrow-derived cells did not contribute to the regeneration of liver vasculature after two-thirds partial hepatectomy (PHx). Taken together, the data reconcile many of the discrepancies in the literature and highlight that the cellular source of regenerating endothelium depends on the fitness of the residual vasculature.
© 2018 Singhal et al.
Figures

Comment in
-
Pumping blood with self-reliance and cooperation.J Exp Med. 2018 Oct 1;215(10):2480-2482. doi: 10.1084/jem.20181537. Epub 2018 Sep 18. J Exp Med. 2018. PMID: 30228158 Free PMC article.
Similar articles
-
Reevaluation of bone marrow-derived cells as a source for hepatocyte regeneration.Cell Transplant. 2004;13(6):659-66. doi: 10.3727/000000004783983521. Cell Transplant. 2004. PMID: 15648736
-
Granulocyte-colony stimulating factor promotes liver repair and induces oval cell migration and proliferation in rats.Gastroenterology. 2007 Aug;133(2):619-31. doi: 10.1053/j.gastro.2007.05.018. Epub 2007 May 21. Gastroenterology. 2007. PMID: 17681181 Free PMC article.
-
Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats.J Clin Invest. 2012 Apr;122(4):1567-73. doi: 10.1172/JCI58789. Epub 2012 Mar 12. J Clin Invest. 2012. PMID: 22406533 Free PMC article.
-
Role of shear stress and immune responses in liver regeneration after a partial hepatectomy.Surg Today. 1999;29(1):1-9. doi: 10.1007/BF02482962. Surg Today. 1999. PMID: 9934824 Review.
-
The role of granulocyte colony-stimulating factor in mobilization and transplantation of peripheral blood progenitor and stem cells.Cytokines Mol Ther. 1995 Dec;1(4):249-70. Cytokines Mol Ther. 1995. PMID: 9384679 Review.
Cited by
-
The Formation of Coronary Vessels in Cardiac Development and Disease.Cold Spring Harb Perspect Biol. 2020 May 1;12(5):a037168. doi: 10.1101/cshperspect.a037168. Cold Spring Harb Perspect Biol. 2020. PMID: 31636078 Free PMC article. Review.
-
Bacillus velezensis HBXN2020 alleviates Salmonella Typhimurium infection in mice by improving intestinal barrier integrity and reducing inflammation.Elife. 2024 Nov 19;13:RP93423. doi: 10.7554/eLife.93423. Elife. 2024. PMID: 39560359 Free PMC article.
-
A perfusion-independent high-throughput method to isolate liver sinusoidal endothelial cells.Commun Biol. 2025 Jan 8;8(1):22. doi: 10.1038/s42003-025-07458-5. Commun Biol. 2025. PMID: 39779980 Free PMC article.
-
Id1 and Id3 Maintain Steady-State Hematopoiesis by Promoting Sinusoidal Endothelial Cell Survival and Regeneration.Cell Rep. 2020 Apr 28;31(4):107572. doi: 10.1016/j.celrep.2020.107572. Cell Rep. 2020. PMID: 32348770 Free PMC article.
-
Angiodiversity and organotypic functions of sinusoidal endothelial cells.Angiogenesis. 2021 May;24(2):289-310. doi: 10.1007/s10456-021-09780-y. Epub 2021 Mar 21. Angiogenesis. 2021. PMID: 33745018 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

