Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation
- PMID: 30194266
- PMCID: PMC6170174
- DOI: 10.1084/jem.20180483
Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation
Abstract
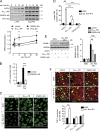
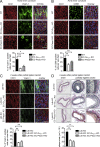
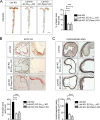
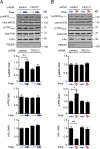
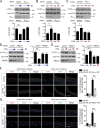
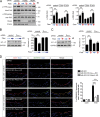
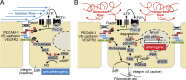
The vascular endothelium is constantly exposed to mechanical forces, including fluid shear stress exerted by the flowing blood. Endothelial cells can sense different flow patterns and convert the mechanical signal of laminar flow into atheroprotective signals, including eNOS activation, whereas disturbed flow in atheroprone areas induces inflammatory signaling, including NF-κB activation. How endothelial cells distinguish different flow patterns is poorly understood. Here we show that both laminar and disturbed flow activate the same initial pathway involving the mechanosensitive cation channel Piezo1, the purinergic P2Y2 receptor, and Gq/G11-mediated signaling. However, only disturbed flow leads to Piezo1- and Gq/G11-mediated integrin activation resulting in focal adhesion kinase-dependent NF-κB activation. Mice with induced endothelium-specific deficiency of Piezo1 or Gαq/Gα11 show reduced integrin activation, inflammatory signaling, and progression of atherosclerosis in atheroprone areas. Our data identify critical steps in endothelial mechanotransduction, which distinguish flow pattern-dependent activation of atheroprotective and atherogenic endothelial signaling and suggest novel therapeutic strategies to treat inflammatory vascular disorders such as atherosclerosis.
© 2018 Albarrán-Juárez et al.
Figures

References
-
- Abramoff M.D., Magalhaes P.J., and Ram S.J.. 2004. Image Processing with ImageJ. Biophoton. Int. 11:36–42.
-
- Baeyens N., Mulligan-Kehoe M.J., Corti F., Simon D.D., Ross T.D., Rhodes J.M., Wang T.Z., Mejean C.O., Simons M., Humphrey J., and Schwartz M.A.. 2014. Syndecan 4 is required for endothelial alignment in flow and atheroprotective signaling. Proc. Natl. Acad. Sci. USA. 111:17308–17313. 10.1073/pnas.1413725111 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

