Biosorption optimization, characterization, immobilization and application of Gelidium amansii biomass for complete Pb2+ removal from aqueous solutions
- PMID: 30194341
- PMCID: PMC6128825
- DOI: 10.1038/s41598-018-31660-7
Biosorption optimization, characterization, immobilization and application of Gelidium amansii biomass for complete Pb2+ removal from aqueous solutions
Abstract
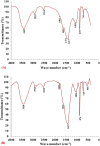
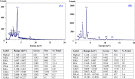
Lead (Pb2+) is among the most toxic heavy metals even in low concentration and cause toxicity to human's health and other forms of life. It is released into the environment through different industrial activities. The biosorption of Pb2+ from aqueous solutions by biomass of commonly available, marine alga Gelidium amansii was studied. The effects of different variables on Pb2+ removal were estimated by a two-level Plackett-Burman factorial design to determine the most significant variables affecting Pb2+ removal % from aqueous solutions. Initial pH, Pb2+ concentration and temperature were the most significant factors affecting Pb2+ removal chosen for further optimization using rotatable central composite design. The maximum removal percentage (100%) of Pb2+ from aqueous solution by Gelidium amansii biomass was found under the optimum conditions: initial Pb2+ concentration of 200 mg/L, temperature 45 °C, pH 4.5, Gelidium amansii biomass of 1 g/L and contact time of 60 minutes at static condition. FTIR analysis of algal biomass revealed the presence of carbonyl, methylene, phosphate, carbonate and phenolic groups, which are involved in the Pb2+ ions biosorption process. SEM analysis demonstrates the ability of Gelidium amansii biomass to adsorb and removes Pb2+ from aqueous solution. EDS analysis shows the additional optical absorption peak corresponding to the Pb2+ which confirms the involvement of Gelidium amansii biomass in the adsorption of Pb2+ ions from aqueous solution. Immobilized Gelidium amansii biomass was effective in Pb2+ removal (100%) from aqueous solution at an initial concentration of 200 mg/L for 3 h. In conclusion, it is demonstrated that the red marine alga Gelidium amansii biomass is a promising, efficient, ecofriendly, cost-effective and biodegradable biosorbent for the removal of Pb2+ from the environment and wastewater effluents.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Volesky, B. Biosorption of heavy metals. (Florida: CRC Press, 2000).
-
- Garbisu C, Alkorta I. Basic concepts on heavy metal soil bioremediation. Eur J Min Proc Environ Protect. 2003;3:58–66.
-
- Alloway, B. J. & Ayres, D. C. Chemical principles of environmental pollution, second edition. Blackie academic and professional. An imprint of chapman and hall, Oxford, London, (1993).
-
- Aksu, Z. Biosorption of heavy metals by micro algae in batch and continuous systems. In: Wong, Y. S., Tam, N. F. Y.(eds) Wastewater treatment with algae. Biotechnology intelligence unit. Springer, Berlin, Heidelberg 37–53 (1998).
-
- WHO. Guidelines for drinking-water quality, third edition. World Health Organisation, Geneva, Switzerland (2004).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

