Liquid-state quantitative SERS analyzer on self-ordered metal liquid-like plasmonic arrays
- PMID: 30194348
- PMCID: PMC6128918
- DOI: 10.1038/s41467-018-05920-z
Liquid-state quantitative SERS analyzer on self-ordered metal liquid-like plasmonic arrays
Abstract
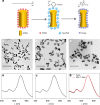
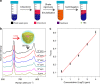
Liquid interfacial plasmonic platform is emerging for new sensors, catalysis, and tunable optical devices, but also promises an alternative for practical applications of surface-enhanced Raman spectroscopy (SERS). Here we show that vigorous mixing of chloroform with citrate-capped gold nanorod sols triggers the rapid self-assembly of three-dimensional plasmonic arrays at the chloroform/water (O/W) interface and produces a self-healing metal liquid-like brilliant golden droplet. The O phase itself generates stable SERS fingerprints and is a good homogeneous internal standard for quantitative analysis. This platform presents reversible O/W encasing in a common cuvette determined just by surface wettability of the container. Both O-in-W and W-in-O platforms exhibit excellent SERS sensitivity and reproducibility for different analytes by the use of a portable Raman device. It paves the way toward a practical and quantitative liquid-state SERS analyzer, likened to a simple UV-Vis spectrometer, that is far superior to typical solid substrate-based or nanoparticle sol-based analysis.
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Jiang YF, Sun DW, Pu HB, Wei QY. Surface enhanced Raman spectroscopy (SERS): a novel reliable technique for rapid detection of common harmful chemical residues. Trend Food Sci. Technol. 2018;75:10–22. doi: 10.1016/j.tifs.2018.02.020. - DOI
-
- Ding SY, et al. Nanostructure-based plasmon-enhanced Raman spectroscopy for surface analysis of materials. Nat. Rev. Mater. 2016;1:16. doi: 10.1038/natrevmats.2016.21. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

