Clinical efficacy of eplerenone versus placebo for central serous chorioretinopathy: study protocol for the VICI randomised controlled trial
- PMID: 30194380
- PMCID: PMC6367466
- DOI: 10.1038/s41433-018-0212-2
Clinical efficacy of eplerenone versus placebo for central serous chorioretinopathy: study protocol for the VICI randomised controlled trial
Abstract
Aims: Chronic central serous chorioretinopathy (CSCR) is poorly understood. Fluid accumulates in the subretinal space and retinal pigment epitheliopathy and neurosensory atrophy may develop. Permanent vision loss occurs in approximately one third of cases. There are no effective treatments for CSCR. Recent studies have shown the mineralocorticoid receptor antagonist, eplerenone, to be effective in resolving subretinal fluid and improving visual acuity. This trial aims to compare the safety and efficacy of eplerenone in patients with CSCR in a double-masked randomised placebo-controlled trial.
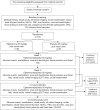
Methods: Patients are randomised 1:1 to receive eplerenone with usual care or placebo with usual care for 12 months; 25 mg per day for 1 week, then 50 mg per day up to 12 months (unless discontinued for safety or resolution of CSCR). Key eligibility criteria are: age 18-60 years, one eye with CSCR for ≥4 months duration, best-corrected visual acuity (BCVA) >53 and <86 letters and no previous treatment. The primary outcome is BCVA at 12 months. Secondary outcomes include resolution of subretinal fluid, development of macular atrophy, subfoveal choroidal thickness, changes in low luminance visual acuity, health-related quality of life and safety.
Conclusions: Recruitment is complete but was slower than expected. We maintained the eligibility criteria to ensure participants had 'true' CSCR and recruited additional centres. Effective distribution of the investigational medicinal product (IMP) was achieved by implementing a database to manage ordering and accountability of IMP packs. The results will provide adequately powered evidence to inform clinical decisions about using eplerenone to treat patients with CSCR.
Conflict of interest statement
Conflict of interest
AW, LC, LE, CAR, AC, SE and BCR have no conflicts of interest. FB-C is an inventor on a patent protecting the use of MR antagonists in CSCR. SS has received research grants, travel grants, and speaker fees from Novartis, Bayer, Allergan, Roche, Heidelberg Engineering, and Optos. AL has received travel grants and speaker fees from Bayer and Roche.
Disclaimer
The views expressed in this publication are those of the author(s) and not necessarily those of the MRC, NHS, NIHR or the Department of Health.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

