Girdin/GIV regulates collective cancer cell migration by controlling cell adhesion and cytoskeletal organization
- PMID: 30194792
- PMCID: PMC6215880
- DOI: 10.1111/cas.13795
Girdin/GIV regulates collective cancer cell migration by controlling cell adhesion and cytoskeletal organization
Abstract
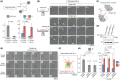
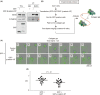
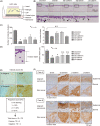
Pathological observations show that cancer cells frequently invade the surrounding stroma in collective groups rather than through single cell migration. Here, we studied the role of the actin-binding protein Girdin, a specific regulator of collective migration of neuroblasts in the brain, in collective cancer cell migration. We found that Girdin was essential for the collective migration of the skin cancer cell line A431 on collagen gels as well as their fibroblast-led collective invasion in an organotypic culture model. We provide evidence that Girdin binds to β-catenin that plays important roles in the Wnt signaling pathway and in E-cadherin-mediated cell-cell adhesion. Girdin-depleted cells displayed scattering and impaired E-cadherin-specific cell-cell adhesion. Importantly, Girdin depletion led to impaired cytoskeletal association of the β-catenin complex, which was accompanied by changes in the supracellular actin cytoskeletal organization of cancer cell cohorts on collagen gels. Although the underlying mechanism is unclear, this observation is consistent with the established role of the actin cytoskeletal system and cell-cell adhesion in the collective behavior of cells. Finally, we showed the correlation of the expression of Girdin with that of the components of the E-cadherin complex and the differentiation of human skin cancer. Collectively, our results suggest that Girdin is an important modulator of the collective behavior of cancer cells.
Keywords: Girdin; actin cytoskeleton; cell adhesion; collective invasion; collective migration.
© 2018 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures



References
-
- Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559‐1564. - PubMed
-
- Yang J, Weinberg RA. Epithelial‐mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818‐829. - PubMed
-
- Yilmaz M, Christofori G. EMT, the cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev. 2009;28:15‐33. - PubMed
MeSH terms
Substances
Grants and funding
- 15H04719/The Ministry of Education, Culture, Sports, Science and Technology of Japan
- 26221304/The Ministry of Education, Culture, Sports, Science and Technology of Japan
- Japan Agency for Medical Research and Development, Core Research for Evolutional Science and Technology
- The Kobayashi Foundation for Cancer Research
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

