The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion
- PMID: 30195028
- PMCID: PMC6289674
- DOI: 10.1016/j.bbi.2018.09.004
The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion
Abstract
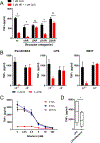
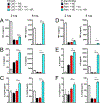
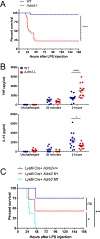
The mammalian nervous system communicates important information about the environment to the immune system, but the underlying mechanisms are largely unknown. Secondary lymphoid organs are highly innervated by sympathetic neurons that secrete norepinephrine (NE) as the primary neurotransmitter. Immune cells express adrenergic receptors, enabling the sympathetic nervous system to directly control immune function. NE is a potent immunosuppressive factor and markedly inhibits TNF-α secretion from innate cells in response to lipopolysaccharide (LPS). In this study, we demonstrate that NE blocks the secretion of a variety of proinflammatory cytokines by rapidly inducing IL-10 secretion from innate cells in response to multiple Toll-like receptor (TLR) signals. NE mediated these effects exclusively through the β2-adrenergic receptor (ADRB2). Consequently, Adrb2-/- animals were more susceptible to L. monocytogenes infection and to intestinal inflammation in a dextran sodium sulfate (DSS) model of colitis. Further, Adrb2-/- animals rapidly succumbed to endotoxemia in response to a sub-lethal LPS challenge and exhibited elevated serum levels of TNF-α and reduced IL-10. LPS-mediated lethality in WT animals was rescued by administering a β 2-specific agonist and in Adrb2-/- animals by exogenous IL-10. These findings reveal a critical role for ADRB2 signaling in controlling inflammation through the rapid induction of IL-10. Our findings provide a fundamental insight into how the sympathetic nervous system controls a critical facet of immune function through ADRB2 signaling.
Keywords: Adrenergic receptor; Inflammation; Interleukin-10; Macrophage; Norepinephrine; Sepsis.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
COMPETING INTERESTS
The authors declare no competing interests.
Figures
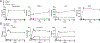


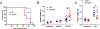

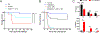
References
-
- Ginhoux F & Jung S Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014). - PubMed
-
- Okabe Y & Medzhitov R Tissue biology perspective on macrophages. Nat. Immunol. 17, 9–17 (2016). - PubMed
-
- Felten DL, Overhage JM, Felten SY & Schmedtje JF Noradrenergic sympathetic innervation of lymphoid tissue in the rabbit appendix: further evidence for a link between the nervous and immune systems. Brain Res. Bull. 7, 595–612 (1981). - PubMed
-
- Williams JM & Felten DL Sympathetic innervation of murine thymus and spleen: a comparative histofluorescence study. Anat. Rec. 199, 531–542 (1981). - PubMed
-
- Williams JM et al. Sympathetic innervation of murine thymus and spleen: evidence for a functional link between the nervous and immune systems. Brain Res. Bull. 6, 83–94 (1981). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

