PDGF Restores the Defective Phenotype of Adipose-Derived Mesenchymal Stromal Cells from Diabetic Patients
- PMID: 30195725
- PMCID: PMC6224797
- DOI: 10.1016/j.ymthe.2018.08.011
PDGF Restores the Defective Phenotype of Adipose-Derived Mesenchymal Stromal Cells from Diabetic Patients
Abstract
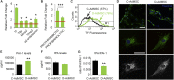
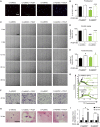
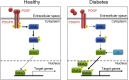
Diabetes is a chronic metabolic disorder that affects 415 million people worldwide. This pathology is often associated with long-term complications, such as critical limb ischemia (CLI), which increases the risk of limb loss and mortality. Mesenchymal stromal cells (MSCs) represent a promising option for the treatment of diabetes complications. Although MSCs are widely used in autologous cell-based therapy, their effects may be influenced by the constant crosstalk between the graft and the host, which could affect the MSC fate potential. In this context, we previously reported that MSCs derived from diabetic patients with CLI have a defective phenotype that manifests as reduced fibrinolytic activity, thereby enhancing the thrombotic risk and compromising patient safety. Here, we found that MSCs derived from diabetic patients with CLI not only exhibit a prothrombotic profile but also have altered multi-differentiation potential, reduced proliferation, and inhibited migration and homing to sites of inflammation. We further demonstrated that this aberrant cell phenotype is reversed by the platelet-derived growth factor (PDGF) BB, indicating that PDGF signaling is a key regulator of MSC functionality. These findings provide an attractive approach to improve the therapeutic efficacy of MSCs in autologous therapy for diabetic patients.
Keywords: PDGF; adipose-derived mesenchymal stromal cells; critical limb ischemia; diabetes; homing; migration; proliferation; thrombotic state.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures





References
-
- Apelqvist J., Bakker K., van Houtum W.H., Nabuurs-Franssen M.H., Schaper N.C., International Working Group on the Diabetic Foot International consensus and practical guidelines on the management and the prevention of the diabetic foot. Diabetes Metab. Res. Rev. 2000;16(Suppl 1):S84–S92. - PubMed
-
- Ruiz-Salmeron R., de la Cuesta-Diaz A., Constantino-Bermejo M., Pérez-Camacho I., Marcos-Sánchez F., Hmadcha A., Soria B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011;20:1629–1639. - PubMed
-
- Kim E.K., Li G., Lee T.J., Hong J.P. The effect of human adipose-derived stem cells on healing of ischemic wounds in a diabetic nude mouse model. Plast. Reconstr. Surg. 2011;128:387–394. - PubMed
-
- Maharlooei M.K., Bagheri M., Solhjou Z., Jahromi B.M., Akrami M., Rohani L., Monabati A., Noorafshan A., Omrani G.R. Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res. Clin. Pract. 2011;93:228–234. - PubMed
-
- Soria B., Montanya E., Martín F., Hmadcha A. A role for the host in the roadmap to diabetes stem cell therapy. Diabetes. 2016;65:1155–1157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

