MiR-663a Stimulates Proliferation and Suppresses Early Apoptosis of Human Spermatogonial Stem Cells by Targeting NFIX and Regulating Cell Cycle
- PMID: 30195770
- PMCID: PMC6037887
- DOI: 10.1016/j.omtn.2018.05.015
MiR-663a Stimulates Proliferation and Suppresses Early Apoptosis of Human Spermatogonial Stem Cells by Targeting NFIX and Regulating Cell Cycle
Abstract
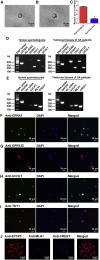
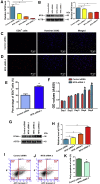
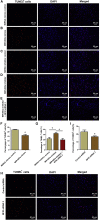
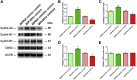
Human spermatogonial stem cells (SSCs) could have significant applications in reproductive medicine and regenerative medicine because of their great plasticity. The fate determinations of human SSCs are mediated by epigenetic factors. However, nothing is known about the regulation of non-coding RNA on human SSCs. Here we have explored for the first time the expression, function, and target of miR-663a in human SSCs. MiR-663a was upregulated in human spermatogonia compared with pachytene spermatocytes, as indicated by microRNA microarray and real-time PCR. CCK-8 and 5-Ethynyl-2'-deoxyuridine (EDU) assays revealed that miR-663a stimulated cell proliferation and DNA synthesis of human SSCs. Annexin V and propidium iodide (PI) staining and flow cytometry demonstrated that miR-663a inhibited early and late apoptosis of human SSCs. Furthermore, NFIX was predicted and verified as a direct target of miR-663a. NFIX silencing led to an enhancement of cell proliferation and DNA synthesis and a reduction of the early apoptosis of human SSCs. NFIX silencing neutralized the influence of miR-663a inhibitor on the proliferation and apoptosis of human SSCs. Finally, both miR-663a mimics and NFIX silencing upregulated the levels of cell cycle regulators, including Cyclin A2, Cyclin B1, and Cyclin E1, whereas miR-663a inhibitor had an adverse effect. Knockdown of Cyclin A2, Cyclin B1, and Cyclin E1 led to the decrease in the proliferation of human SSCs. Collectively, miR-663a has been identified as the first microRNA that promotes the proliferation and DNA synthesis and suppresses the early apoptosis of human SSCs by targeting NFIX via cell cycle regulators Cyclin A2, Cyclin B1, and Cyclin E1. This study thus provides novel insights into the molecular mechanisms underlying human spermatogenesis, and it could offer novel targets for treating male infertility and other human diseases.
Keywords: NFIX; apoptosis; cell cycle proteins; human spermatogonial stem cells; miRNA-663a; proliferation.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures







Similar articles
-
miRNA-31-5p Mediates the Proliferation and Apoptosis of Human Spermatogonial Stem Cells via Targeting JAZF1 and Cyclin A2.Mol Ther Nucleic Acids. 2019 Mar 1;14:90-100. doi: 10.1016/j.omtn.2018.11.004. Epub 2018 Nov 20. Mol Ther Nucleic Acids. 2019. PMID: 30583099 Free PMC article.
-
Hsa-miR-1908-3p Mediates the Self-Renewal and Apoptosis of Human Spermatogonial Stem Cells via Targeting KLF2.Mol Ther Nucleic Acids. 2020 Jun 5;20:788-800. doi: 10.1016/j.omtn.2020.04.016. Epub 2020 May 1. Mol Ther Nucleic Acids. 2020. PMID: 32438314 Free PMC article.
-
miRNA-122-5p stimulates the proliferation and DNA synthesis and inhibits the early apoptosis of human spermatogonial stem cells by targeting CBL and competing with lncRNA CASC7.Aging (Albany NY). 2020 Nov 24;12(24):25528-25546. doi: 10.18632/aging.104158. Epub 2020 Nov 24. Aging (Albany NY). 2020. PMID: 33231565 Free PMC article.
-
The mechanisms and functions of microRNAs in mediating the fate determinations of human spermatogonial stem cells and Sertoli cells.Semin Cell Dev Biol. 2022 Jan;121:32-39. doi: 10.1016/j.semcdb.2021.05.003. Epub 2021 May 24. Semin Cell Dev Biol. 2022. PMID: 34034987 Review.
-
Regulation of long non-coding RNAs and circular RNAs in spermatogonial stem cells.Reproduction. 2019 Jul;158(1):R15-R25. doi: 10.1530/REP-18-0517. Reproduction. 2019. PMID: 30939448 Review.
Cited by
-
Alpha-tocopherol enhances spermatogonial stem cell proliferation and restores mouse spermatogenesis by up-regulating BMI1.Front Nutr. 2023 Apr 17;10:1141964. doi: 10.3389/fnut.2023.1141964. eCollection 2023. Front Nutr. 2023. PMID: 37139440 Free PMC article.
-
Stromal cells downregulate miR-23a-5p to activate protective autophagy in acute myeloid leukemia.Cell Death Dis. 2019 Sep 30;10(10):736. doi: 10.1038/s41419-019-1964-8. Cell Death Dis. 2019. PMID: 31570693 Free PMC article.
-
NFIX suppresses breast cancer cell proliferation by delaying mitosis through downregulation of CDK1 expression.Cell Death Discov. 2025 Feb 25;11(1):77. doi: 10.1038/s41420-025-02361-8. Cell Death Discov. 2025. PMID: 40000619 Free PMC article.
-
Unraveling the Regulatory Function of MicroRNAs in Reproductive System Apoptosis and Their Implications for Infertility.Reprod Sci. 2025 Aug;32(8):2515-2527. doi: 10.1007/s43032-025-01897-5. Epub 2025 Jul 2. Reprod Sci. 2025. PMID: 40603697 Review.
-
The microRNA-mediated apoptotic signaling axis in male reproduction: a possible and targetable culprit in male infertility.Cell Biol Toxicol. 2025 Mar 5;41(1):54. doi: 10.1007/s10565-025-10006-w. Cell Biol Toxicol. 2025. PMID: 40038116 Free PMC article. Review.
References
-
- Kanatsu-Shinohara M., Inoue K., Lee J., Yoshimoto M., Ogonuki N., Miki H., Baba S., Kato T., Kazuki Y., Toyokuni S. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004;119:1001–1012. - PubMed
-
- Zhang Z., Gong Y., Guo Y., Hai Y., Yang H., Yang S., Liu Y., Ma M., Liu L., Li Z. Direct transdifferentiation of spermatogonial stem cells to morphological, phenotypic and functional hepatocyte-like cells via the ERK1/2 and Smad2/3 signaling pathways and the inactivation of cyclin A, cyclin B and cyclin E. Cell Commun. Signal. 2013;11:67. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

