Ribosomal Protein S3 Gene Silencing Protects Against Cigarette Smoke-Induced Acute Lung Injury
- PMID: 30195775
- PMCID: PMC6031153
- DOI: 10.1016/j.omtn.2018.05.027
Ribosomal Protein S3 Gene Silencing Protects Against Cigarette Smoke-Induced Acute Lung Injury
Abstract
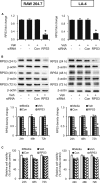
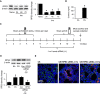
Chronic obstructive pulmonary disease (COPD) is estimated to be the third leading cause of death by 2030. Transcription factor NF-κB may play a critical role in COPD pathogenesis. Ribosomal protein S3 (RPS3), a 40S ribosomal protein essential for executing protein translation, has recently been found to interact with the NF-κB p65 subunit and promote p65 DNA-binding activity. We sought to study whether RPS3 gene silencing could protect against cigarette-smoke (CS)-induced acute lung injury in a mouse model. Effects of an intratracheal RPS3 siRNA in CS-induced lung injury were determined by measuring bronchoalveolar lavage (BAL) fluid cell counts, levels of inflammatory and oxidative damage markers, and NF-κB translocation. Lung RPS3 level was found to be upregulated for the first time with CS exposure, and RPS3 siRNA blocked CS-induced neutrophil counts in BAL fluid. RPS3 siRNA suppressed CS-induced lung inflammatory mediator and oxidative damage marker levels, as well as nuclear p65 accumulation and transcriptional activation. RPS3 siRNA was able to disrupt CS extract (CSE)-induced NF-κB activation in an NF-κB reporter gene assay. We report for the first time that RPS3 gene silencing ameliorated CS-induced acute lung injury, probably via interruption of the NF-κB activity, postulating that RPS3 is a novel therapeutic target for COPD.
Keywords: NF-KB; chronic obstructive pulmonary disease; cigarette smoke; ribosomal protein S3; siRNA.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Gene silencing of receptor-interacting protein 2 protects against cigarette smoke-induced acute lung injury.Pharmacol Res. 2019 Jan;139:560-568. doi: 10.1016/j.phrs.2018.10.016. Epub 2018 Oct 28. Pharmacol Res. 2019. PMID: 30394320
-
Ribosomal protein S3 gene silencing protects against experimental allergic asthma.Br J Pharmacol. 2017 Apr;174(7):540-552. doi: 10.1111/bph.13717. Epub 2017 Feb 24. Br J Pharmacol. 2017. PMID: 28093718 Free PMC article.
-
Identification of an N-terminal truncation of the NF-κB p65 subunit that specifically modulates ribosomal protein S3-dependent NF-κB gene expression.J Biol Chem. 2012 Dec 14;287(51):43019-29. doi: 10.1074/jbc.M112.388694. Epub 2012 Oct 31. J Biol Chem. 2012. PMID: 23115242 Free PMC article.
-
Activation of angiotensin II type-2 receptor protects against cigarette smoke-induced COPD.Pharmacol Res. 2020 Nov;161:105223. doi: 10.1016/j.phrs.2020.105223. Epub 2020 Oct 2. Pharmacol Res. 2020. PMID: 33017650 Free PMC article.
-
Baicalin attenuates inflammation by inhibiting NF-kappaB activation in cigarette smoke induced inflammatory models.Pulm Pharmacol Ther. 2010 Oct;23(5):411-9. doi: 10.1016/j.pupt.2010.05.004. Epub 2010 May 23. Pulm Pharmacol Ther. 2010. PMID: 20566376
Cited by
-
STAT3-activated lncRNA XIST accelerates the inflammatory response and apoptosis of LPS-induced acute lung injury.J Cell Mol Med. 2021 Jul;25(14):6550-6557. doi: 10.1111/jcmm.16653. Epub 2021 Jun 11. J Cell Mol Med. 2021. PMID: 34114724 Free PMC article.
-
Inhaled RNA Therapy: From Promise to Reality.Trends Pharmacol Sci. 2020 Oct;41(10):715-729. doi: 10.1016/j.tips.2020.08.002. Epub 2020 Sep 4. Trends Pharmacol Sci. 2020. PMID: 32893004 Free PMC article. Review.
-
RNAi therapeutic strategies for acute respiratory distress syndrome.Transl Res. 2019 Dec;214:30-49. doi: 10.1016/j.trsl.2019.07.011. Epub 2019 Jul 27. Transl Res. 2019. PMID: 31401266 Free PMC article. Review.
-
Inhaled siRNA Formulations for Respiratory Diseases: From Basic Research to Clinical Application.Pharmaceutics. 2022 Jun 2;14(6):1193. doi: 10.3390/pharmaceutics14061193. Pharmaceutics. 2022. PMID: 35745766 Free PMC article. Review.
-
Identification of biomarkers associated with mitochondrial dysfunction and programmed cell death in chronic obstructive pulmonary disease via transcriptomics.Front Genet. 2025 Jun 19;16:1567173. doi: 10.3389/fgene.2025.1567173. eCollection 2025. Front Genet. 2025. PMID: 40612794 Free PMC article.
References
-
- Barnes P.J. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2016;138:16–27. - PubMed
-
- Barnes P.J. Immunology of asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2008;8:183–192. - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease (2015). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease – 2016. http://goldcopd.org/global-strategy-diagnosis-management-prevention-copd.... - PubMed
-
- Barnes P.J. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat. Rev. Drug Discov. 2013;12:543–559. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

