Long-Term Morpholino Oligomers in Hexose Elicits Long-Lasting Therapeutic Improvements in mdx Mice
- PMID: 30195785
- PMCID: PMC6070676
- DOI: 10.1016/j.omtn.2018.06.005
Long-Term Morpholino Oligomers in Hexose Elicits Long-Lasting Therapeutic Improvements in mdx Mice
Erratum in
-
Erratum: Long-Term Morpholino Oligomers in Hexose Elicit Long-Lasting Therapeutic Improvements in mdx Mice.Mol Ther Nucleic Acids. 2020 Sep 17;22:196-197. doi: 10.1016/j.omtn.2020.09.005. eCollection 2020 Dec 4. Mol Ther Nucleic Acids. 2020. PMID: 33230426 Free PMC article.
Abstract
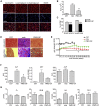
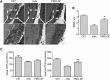
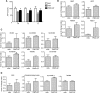
Approval of antisense oligonucleotide eteplirsen highlights the promise of exon-skipping therapeutics for Duchenne muscular dystrophy patients. However, the limited efficacy of eteplirsen underscores the importance to improve systemic delivery and efficacy. Recently, we demonstrated that a glucose and fructose (GF) delivery formulation effectively potentiates phosphorodiamidate morpholino oligomer (PMO). Considering the clinical potential of GF, it is important to determine the long-term compatibility and efficacy with PMO in mdx mice prior to clinical translation. Here, we report that yearlong administration of a clinically applicable PMO dose (50 mg/kg/week for 3 weeks followed by 50 mg/kg/month for 11 months) with GF elicited sustainably high levels of dystrophin expression in mdx mice, with up to 45% of the normal level of dystrophin restored in most peripheral muscles without any detectable toxicity. Importantly, PMO-GF resulted in phenotypical rescue and mitochondrial biogenesis with functional improvement. Carbohydrate metabolites measurements revealed improved metabolic and energetic conditions after PMO-GF treatment in mdx mice without metabolic anomaly. Collectively, our study shows PMO-GF's ability to elicit long-lasting therapeutic effects with tolerable toxicity and represents a new treatment modality for Duchenne muscular dystrophy, and provides guidelines for antisense oligonucleotides with GF in clinical use.
Keywords: Duchenne muscular dystrophy; GF; exon skipping; mitochondria; morpholino oligomers.
Copyright © 2018 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Hexose Potentiates Peptide-Conjugated Morpholino Oligomer Efficacy in Cardiac Muscles of Dystrophic Mice in an Age-Dependent Manner.Mol Ther Nucleic Acids. 2019 Dec 6;18:341-350. doi: 10.1016/j.omtn.2019.09.012. Epub 2019 Sep 23. Mol Ther Nucleic Acids. 2019. PMID: 31629961 Free PMC article.
-
Hexose enhances oligonucleotide delivery and exon skipping in dystrophin-deficient mdx mice.Nat Commun. 2016 Mar 11;7:10981. doi: 10.1038/ncomms10981. Nat Commun. 2016. PMID: 26964641 Free PMC article.
-
Fructose Promotes Uptake and Activity of Oligonucleotides With Different Chemistries in a Context-dependent Manner in mdx Mice.Mol Ther Nucleic Acids. 2016 Jun 28;5(6):e329. doi: 10.1038/mtna.2016.46. Mol Ther Nucleic Acids. 2016. PMID: 27351681 Free PMC article.
-
Cell-penetrating peptide-morpholino conjugates alter pre-mRNA splicing of DMD (Duchenne muscular dystrophy) and inhibit murine coronavirus replication in vivo.Biochem Soc Trans. 2007 Aug;35(Pt 4):826-8. doi: 10.1042/BST0350826. Biochem Soc Trans. 2007. PMID: 17635157 Review.
-
Multiple Exon Skipping in the Duchenne Muscular Dystrophy Hot Spots: Prospects and Challenges.J Pers Med. 2018 Dec 7;8(4):41. doi: 10.3390/jpm8040041. J Pers Med. 2018. PMID: 30544634 Free PMC article. Review.
Cited by
-
MiR-199-3p enhances muscle regeneration and ameliorates aged muscle and muscular dystrophy.Commun Biol. 2021 Mar 29;4(1):427. doi: 10.1038/s42003-021-01952-2. Commun Biol. 2021. PMID: 33782502 Free PMC article.
-
Advances in Duchenne Muscular Dystrophy: Diagnostic Techniques and Dystrophin Domain Insights.Int J Mol Sci. 2025 Apr 10;26(8):3579. doi: 10.3390/ijms26083579. Int J Mol Sci. 2025. PMID: 40332074 Free PMC article. Review.
-
Control of backbone chemistry and chirality boost oligonucleotide splice switching activity.Nucleic Acids Res. 2022 Jun 10;50(10):5443-5466. doi: 10.1093/nar/gkac018. Nucleic Acids Res. 2022. PMID: 35061895 Free PMC article.
-
In vivo restoration of dystrophin expression in mdx mice using intra-muscular and intra-arterial injections of hydrogel microsphere carriers of exon skipping antisense oligonucleotides.Cell Death Dis. 2022 Sep 9;13(9):779. doi: 10.1038/s41419-022-05166-0. Cell Death Dis. 2022. PMID: 36085138 Free PMC article.
-
Resolution of fibrosis in mdx dystrophic mouse after oral consumption of N-163 strain of Aureobasidium pullulans produced β-glucan.Sci Rep. 2023 Oct 9;13(1):17008. doi: 10.1038/s41598-023-44330-0. Sci Rep. 2023. PMID: 37813938 Free PMC article.
References
-
- Mendell J.R., Rodino-Klapac L.R., Sahenk Z., Roush K., Bird L., Lowes L.P., Alfano L., Gomez A.M., Lewis S., Kota J., Eteplirsen Study Group Eteplirsen for the treatment of Duchenne muscular dystrophy. Ann. Neurol. 2013;74:637–647. - PubMed
-
- Falzarano M.S., Passarelli C., Bassi E., Fabris M., Perrone D., Sabatelli P., Maraldi N.M., Donà S., Selvatici R., Bonaldo P. Biodistribution and molecular studies on orally administered nanoparticle-AON complexes encapsulated with alginate aiming at inducing dystrophin rescue in mdx mice. BioMed Res. Int. 2013;2013:527418. - PMC - PubMed
-
- Rimessi P., Sabatelli P., Fabris M., Braghetta P., Bassi E., Spitali P., Vattemi G., Tomelleri G., Mari L., Perrone D. Cationic PMMA nanoparticles bind and deliver antisense oligoribonucleotides allowing restoration of dystrophin expression in the mdx mouse. Mol. Ther. 2009;17:820–827. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

