The Use of Somatic Hypermutation for the Affinity Maturation of Therapeutic Antibodies
- PMID: 30196512
- PMCID: PMC6497467
- DOI: 10.1007/978-1-4939-8648-4_24
The Use of Somatic Hypermutation for the Affinity Maturation of Therapeutic Antibodies
Abstract
The engineering of antibodies and antibody fragments for affinity maturation, stability, and other biophysical characteristics is a common aspect of therapeutic development. Maturation of antibodies in B cells during the adaptive immune response is the result of a process called somatic hypermutation (SHM), in which the activation-induced cytidine deaminase (AID) acts to introduce mutations into immunoglobulin (Ig) genes. Iterative selection and clonal expansion of B cells containing affinity-enhancing mutations drive an increase in the overall affinity of antibodies. Here we describe the use of SHM coupled with mammalian cell surface display for the maturation of antibodies in vitro and the complementarity of these methods with the mining of immune lineages using next-generation sequencing (NGS).
Keywords: Affinity maturation; Antibody; Complementarity-determining region (CDR); Fluorescence-activated cell sorting (FACS); Heavy chain (HC); Light chain (LC); Somatic hypermutation (SHM).
Figures

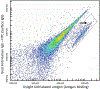
References
-
- Tas JM, Mesin L, Pasqual G, Targ S, Jacobsen JT, Mano YM, Chen CS, Weill JC, Reynaud CA, Browne EP, Meyer-Hermann M, Victora GD (2016) Visualizing antibody affinity maturation in germinal centers. Science 351(6277): 1048–1054. https://doi.org/10.1126/science.aad3439 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

