Follistatin supplementation during in vitro embryo culture improves developmental competence of bovine embryos produced using sex-sorted semen
- PMID: 30196810
- PMCID: PMC7747478
- DOI: 10.1016/j.repbio.2018.06.004
Follistatin supplementation during in vitro embryo culture improves developmental competence of bovine embryos produced using sex-sorted semen
Abstract
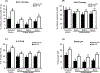
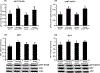
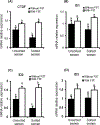
Using sex-sorted semen to produce offspring of desired sex is associated with reduced developmental competence in vitro and lower fertility rates in vivo. The objectives of the present study were to investigate the effects of exogenous follistatin supplementation on the developmental competence of bovine embryos produced with sex-sorted semen and possible link between TGF-β regulated pathways and embryotrophic actions of follistatin. Effects of follistatin on expression of cell lineage markers (CDX2 and Nanog) and downstream targets of SMAD signaling (CTGF, ID1, ID2 and ID3) and AKT phosphorylation were investigated. Follistatin was supplemented during the initial 72 h of embryo culture. Exogenous follistatin restored the in vitro developmental competence of embryos produced with sex-sorted semen to the levels of control embryos produced with unsorted semen, and comparable results were obtained using sorted semen from three different bulls. The mRNA abundance for SMAD signaling downstream target genes, CTGF (SMAD 2/3 pathway) and ID2 (SMAD 1/5 pathway), was lower in blastocysts produced using sex-sorted versus unsorted semen, but mRNA levels for CDX2, NANOG, ID1 and ID3 were similar in both groups. Follistatin supplementation restored CTGF and ID2 mRNA in blastocysts produced using sex-sorted semen to levels of control embryos. Moreover, levels of phosphorylated (p)AKT (Ser-473 and Thr-308) were similar in embryos derived from sex-sorted and unsorted semen, but follistatin treatment increased pAKT levels in both groups. Taken together, results demonstrated that follistatin improves in vitro development of embryos produced with sex-sorted semen and such effects are associated with enhanced indices of SMAD signaling.
Keywords: AKT; Bovine; Follistatin; Oocytes quality; SMAD; Sorted semen; TGF-β.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest statement
None of the authors has any financial or other potential conflict of interest related to this manuscript.
Figures
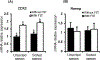
Similar articles
-
Follistatin supplementation induces changes in CDX2 CpG methylation and improves in vitro development of bovine SCNT preimplantation embryos.Reprod Biol Endocrinol. 2021 Sep 13;19(1):141. doi: 10.1186/s12958-021-00829-7. Reprod Biol Endocrinol. 2021. PMID: 34517901 Free PMC article.
-
Production of female bovine embryos with sex-sorted sperm using intracytoplasmic sperm injection: efficiency and in vitro developmental competence.Theriogenology. 2014 Mar 15;81(5):675-82.e1. doi: 10.1016/j.theriogenology.2013.11.010. Epub 2013 Nov 21. Theriogenology. 2014. PMID: 24360289
-
Developmental kinetics and viability of bovine embryos produced in vitro with sex-sorted semen.Theriogenology. 2021 Feb;161:243-251. doi: 10.1016/j.theriogenology.2020.12.001. Epub 2020 Dec 9. Theriogenology. 2021. PMID: 33340757
-
Effects of gamete source and culture conditions on the competence of in vitro-produced embryos for post-transfer survival in cattle.Reprod Fertil Dev. 2010;22(1):59-66. doi: 10.1071/RD09212. Reprod Fertil Dev. 2010. PMID: 20003846 Review.
-
State-of-the-art embryo technologies in cattle.Soc Reprod Fertil Suppl. 2007;64:315-25. doi: 10.5661/rdr-vi-315. Soc Reprod Fertil Suppl. 2007. PMID: 17491156 Review.
Cited by
-
High-Efficiency Bovine Sperm Sexing Used Magnetic-Activated Cell Sorting by Coupling scFv Antibodies Specific to Y-Chromosome-Bearing Sperm on Magnetic Microbeads.Biology (Basel). 2022 May 6;11(5):715. doi: 10.3390/biology11050715. Biology (Basel). 2022. PMID: 35625442 Free PMC article.
-
Follistatin treatment modifies DNA methylation of the CDX2 gene in bovine preimplantation embryos.Mol Reprod Dev. 2020 Sep;87(9):998-1008. doi: 10.1002/mrd.23409. Epub 2020 Aug 10. Mol Reprod Dev. 2020. PMID: 32776625 Free PMC article.
-
Follistatin supplementation induces changes in CDX2 CpG methylation and improves in vitro development of bovine SCNT preimplantation embryos.Reprod Biol Endocrinol. 2021 Sep 13;19(1):141. doi: 10.1186/s12958-021-00829-7. Reprod Biol Endocrinol. 2021. PMID: 34517901 Free PMC article.
-
Bioactive supplements influencing bovine in vitro embryo development.J Anim Sci. 2022 Jul 1;100(7):skac091. doi: 10.1093/jas/skac091. J Anim Sci. 2022. PMID: 35772761 Free PMC article. Review.
References
-
- Wheeler MB, Rutledge JJ, Fischer-Brown A, VanEtten T, Malusky S, Beebe DJ. Application of sexed semen technology to in vitro embryo production in cattle. Theriogenology 2006;65:219–27. - PubMed
-
- Hossein-Zadeh NG, Nejati-Javaremi A, Miraei-Ashtiani SR, Kohram H. Bio-economic evaluation of the use of sexed semen at different conception rates and herd sizes in Holstein populations. Anim Reprod Sci 2010;121:17–23. - PubMed
-
- Trigal B, Gomez E, Caamano JN, Munoz M, Moreno J, Carrocera S, et al. In vitro and in vivo quality of bovine embryos in vitro produced with sex-sorted sperm. Theriogenology 2012;78:1465–75. - PubMed
-
- Xu J, Chaubal SA, Du F. Optimizing IVF with sexed sperm in cattle. Theriogenology 2009;71:39–47. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

