Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery
- PMID: 30196853
- PMCID: PMC6127971
- DOI: 10.1016/j.ymthe.2018.05.025
Retinal AAV8-RS1 Gene Therapy for X-Linked Retinoschisis: Initial Findings from a Phase I/IIa Trial by Intravitreal Delivery
Abstract
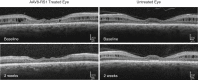
This study evaluated the safety and tolerability of ocular RS1 adeno-associated virus (AAV8-RS1) gene augmentation therapy to the retina of participants with X-linked retinoschisis (XLRS). XLRS is a monogenic trait affecting only males, caused by mutations in the RS1 gene. Retinoschisin protein is secreted principally in the outer retina, and its absence results in retinal cavities, synaptic dysfunction, reduced visual acuity, and susceptibility to retinal detachment. This phase I/IIa single-center, prospective, open-label, three-dose-escalation clinical trial administered vector to nine participants with pathogenic RS1 mutations. The eye of each participant with worse acuity (≤63 letters; Snellen 20/63) received the AAV8-RS1 gene vector by intravitreal injection. Three participants were assigned to each of three dosage groups: 1e9 vector genomes (vg)/eye, 1e10 vg/eye, and 1e11 vg/eye. The investigational product was generally well tolerated in all but one individual. Ocular events included dose-related inflammation that resolved with topical and oral corticosteroids. Systemic antibodies against AAV8 increased in a dose-related fashion, but no antibodies against RS1 were observed. Retinal cavities closed transiently in one participant. Additional doses and immunosuppressive regimens are being explored to pursue evidence of safety and efficacy (ClinicalTrials.gov: NCT02317887).
Keywords: AAV vector; X-linked retinoschisis; clinical trial; gene therapy; ocular disease; retinal disease.
Copyright © 2018. Published by Elsevier Inc.
Figures

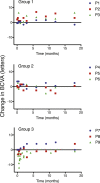
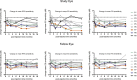
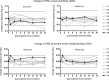
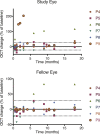
Comment in
-
Breaking and Sealing Barriers in Retinal Gene Therapy.Mol Ther. 2018 Sep 5;26(9):2081-2082. doi: 10.1016/j.ymthe.2018.08.003. Epub 2018 Aug 11. Mol Ther. 2018. PMID: 30107998 Free PMC article. No abstract available.
References
-
- de la Chapelle A., Hästbacka J., Lehesjoki A.E., Sulisalo T., Kere J., Tahvanainen E., Sistonen P. [Linkage and linkage disequilibrium in the Finnish disease heritage] Duodecim. 1994;110:654–664. - PubMed
-
- Puech B., Kostrubiec B., Hache J.C., François P. [Epidemiology and prevalence of hereditary retinal dystrophies in the Northern France] J. Fr. Ophtalmol. 1991;14:153–164. - PubMed
-
- Skoog K.O., Textorius O., Nilsson S.E. Evaluation of patients with obvious or suspected degenerative and dystrophic disorders of the retina, pigment epithelium and choroid. Experience from a Swedish referral center. Acta Ophthalmol. (Copenh.) 1990;68:131–138. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

